Abstract
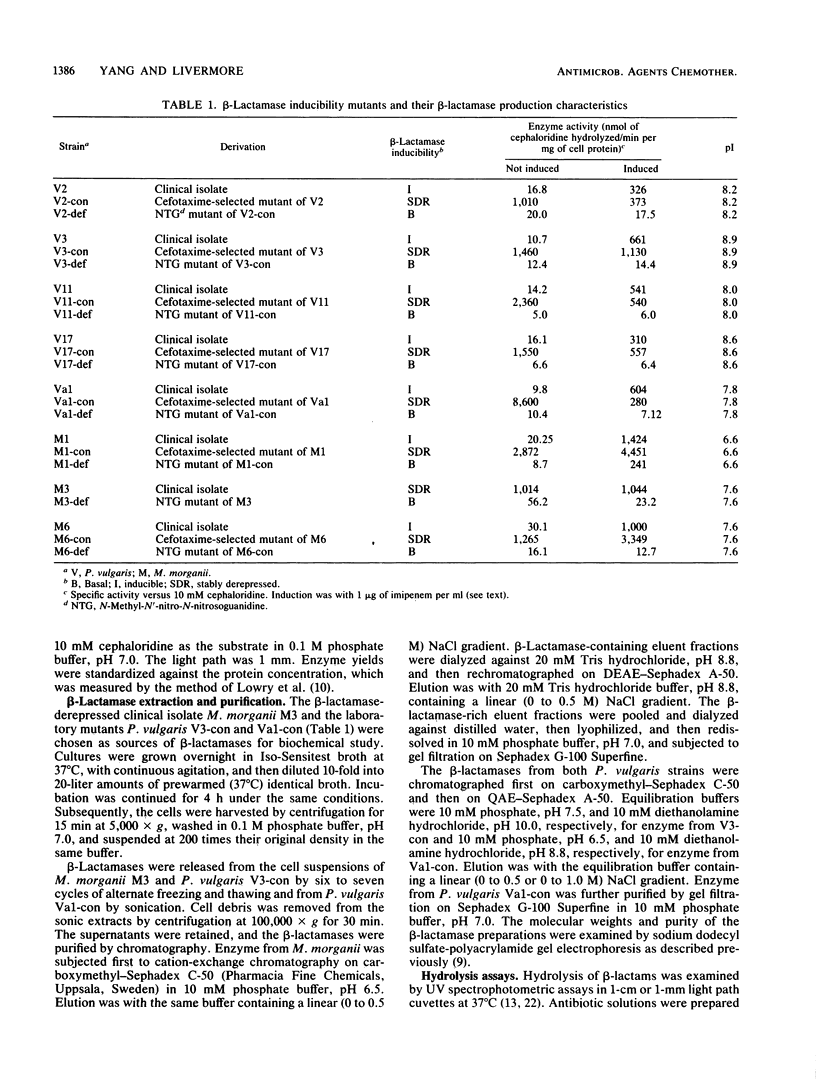
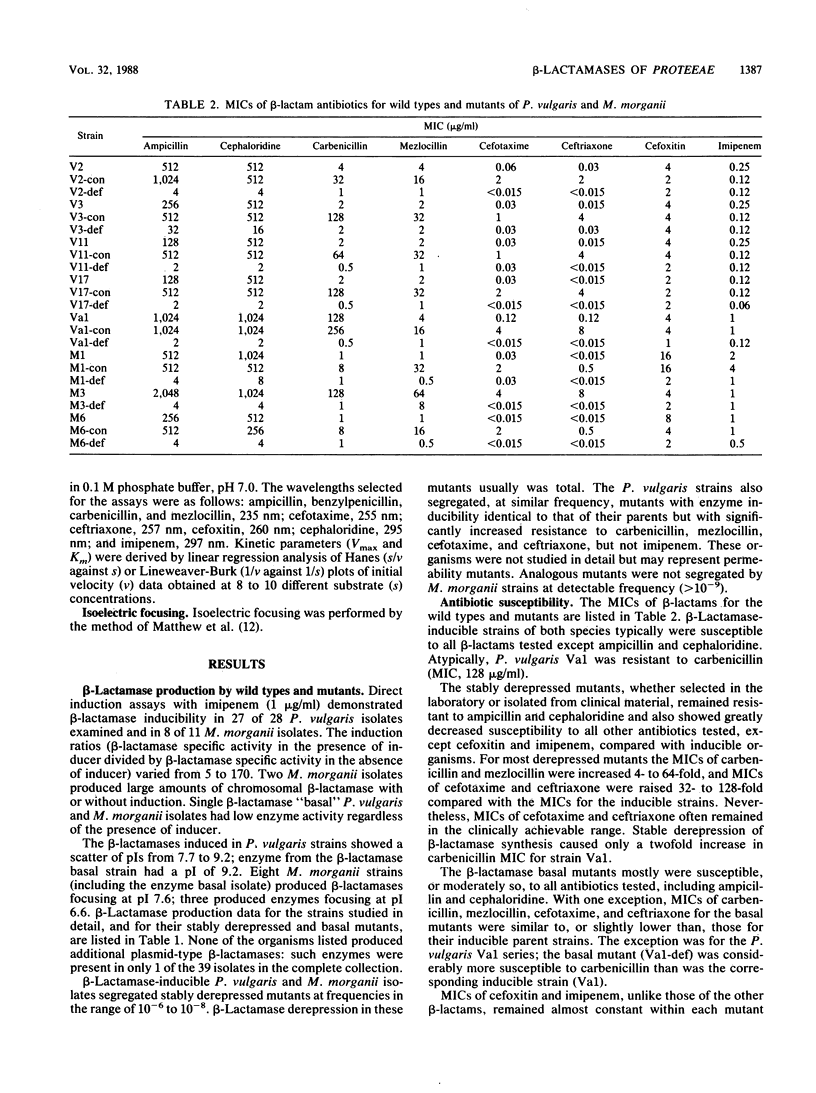
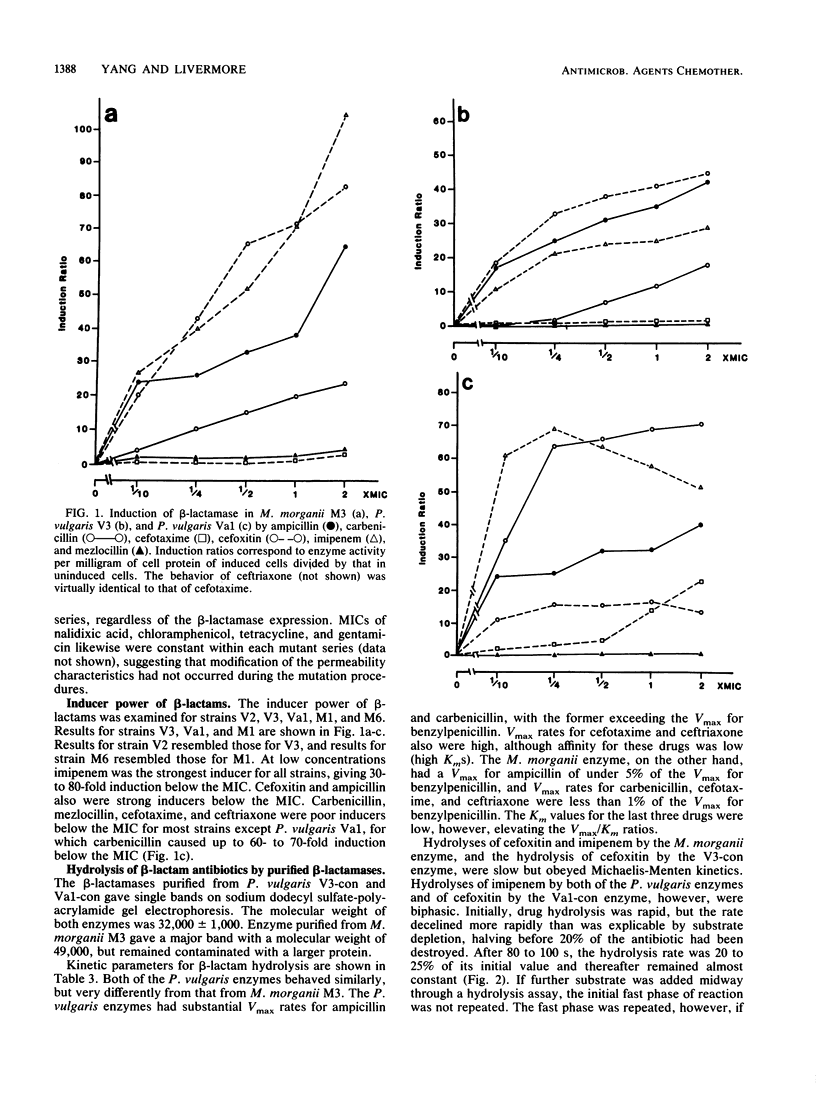
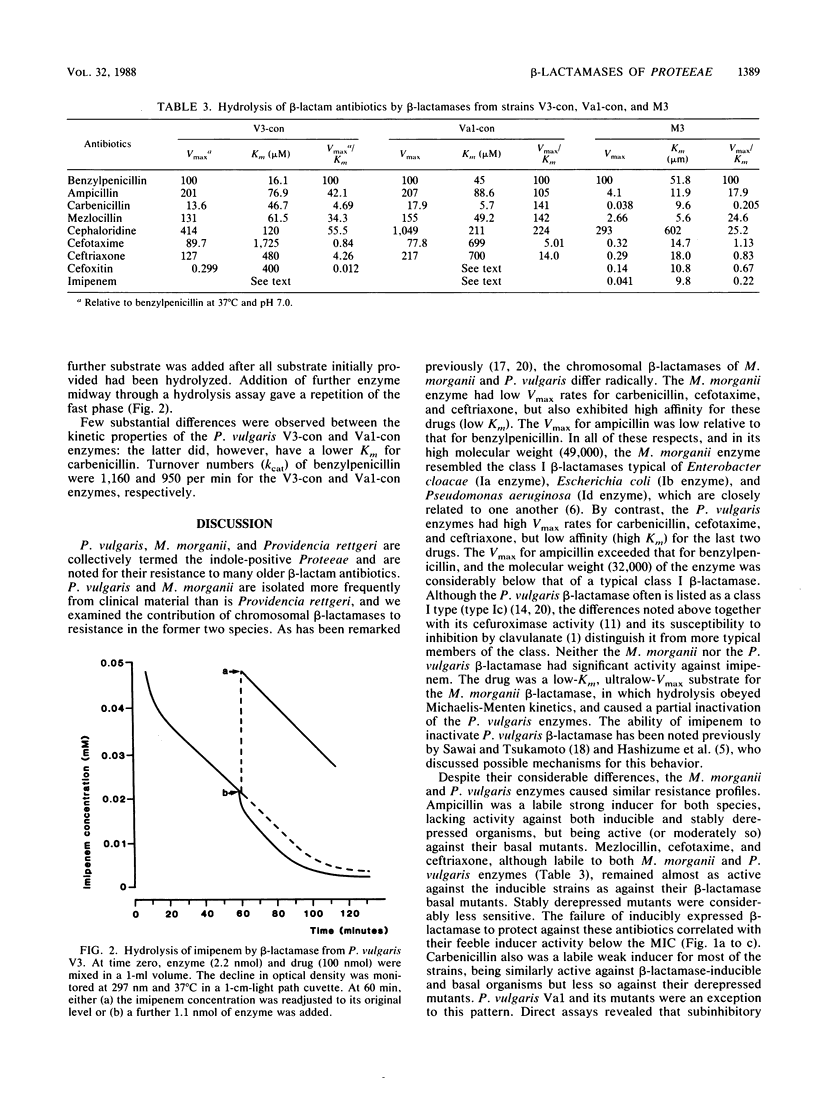
Indole-positive members of the Proteeae usually have inducible expression of chromosomal beta-lactamases. Mutants with stably derepressed beta-lactamase expression occur in inducible populations at frequencies in the range of 10(-6) to 10(-8). The contribution of these beta-lactamases to drug resistance was examined in Morganella morganii and Proteus vulgaris. The M. morganii enzyme was a high-molecular-weight (49,000) class I cephalosporinase with low Vmax rates for ampicillin, carbenicillin, and and broad-spectrum cephalosporins. The P. vulgaris enzyme had a lower molecular weight (32,000) and high Vmax rates for ampicillin, cephaloridine, cefotaxime, and ceftriaxone. Imipenem and cefoxitin inactivated the P. vulgaris enzyme but were low-Vmax, low-Km substrates for that of M. morganii. Despite these differences, the two beta-lactamases caused similar resistance profiles. Ampicillin and cephaloridine were strong inducers for both species, and beta-lactamase-inducible strains and their stably derepressed mutants were resistant, whereas basal mutants (those with low-level uninducible beta-lactamase) were susceptible to these two compounds. Mezlocillin, cefotaxime, ceftriaxone, and (usually) carbenicillin were almost equally active against beta-lactamase-inducible organisms and their basal mutants, but were less active against stably derepressed mutants. This behavior reflected the beta-lactamase lability of these drugs, coupled with their weak inducer activity below the MIC. Carbenicillin was a labile strong inducer for a single P. vulgaris strain, and inducible enzyme was protective against the drug in this atypical organism. Cefoxitin and imipenem, both strong inducers below the MIC, were almost equally active against beta-lactamase-inducible organisms and their basal and stably derepressed mutants.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aspiotis A., Cullmann W., Dick W., Stieglitz M. Inducible beta-lactamases are principally responsible for the naturally occurring resistance towards beta-lactam antibiotics in Proteus vulgaris. Chemotherapy. 1986;32(3):236–246. doi: 10.1159/000238420. [DOI] [PubMed] [Google Scholar]
- Curtis N. A., Brown C., Boxall M., Boulton M. G. Modified peptidoglycan transpeptidase activity in a carbenicillin-resistant mutant of Pseudomonas aeruginosa 18s. Antimicrob Agents Chemother. 1978 Aug;14(2):246–251. doi: 10.1128/aac.14.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N. A., Eisenstadt R. L., Rudd C., White A. J. Inducible type I beta-lactamases of gram-negative bacteria and resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1986 Jan;17(1):51–61. doi: 10.1093/jac/17.1.51. [DOI] [PubMed] [Google Scholar]
- Dworzack D. L., Pugsley M. P., Sanders C. C., Horowitz E. A. Emergence of resistance in gram-negative bacteria during therapy with expanded-spectrum cephalosporins. Eur J Clin Microbiol. 1987 Aug;6(4):456–459. doi: 10.1007/BF02013110. [DOI] [PubMed] [Google Scholar]
- Hashizume T., Yamaguchi A., Hirata T., Sawai T. Kinetic studies on the inhibition of Proteus vulgaris beta-lactamase by imipenem. Antimicrob Agents Chemother. 1984 Jan;25(1):149–151. doi: 10.1128/aac.25.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris B., Dusart J., Frere J. M., van Beeumen J., Emanuel E. L., Petursson S., Gagnon J., Waley S. G. The active site of the P99 beta-lactamase from Enterobacter cloacae. Biochem J. 1984 Oct 1;223(1):271–274. doi: 10.1042/bj2230271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Livermore D. M. Clinical significance of beta-lactamase induction and stable derepression in gram-negative rods. Eur J Clin Microbiol. 1987 Aug;6(4):439–445. doi: 10.1007/BF02013107. [DOI] [PubMed] [Google Scholar]
- Livermore D. M. Do beta-lactamases 'trap' cephalosporins? J Antimicrob Chemother. 1985 May;15(5):511–514. doi: 10.1093/jac/15.5.511. [DOI] [PubMed] [Google Scholar]
- Livermore D. M., Yang Y. J. Beta-lactamase lability and inducer power of newer beta-lactam antibiotics in relation to their activity against beta-lactamase-inducibility mutants of Pseudomonas aeruginosa. J Infect Dis. 1987 Apr;155(4):775–782. doi: 10.1093/infdis/155.4.775. [DOI] [PubMed] [Google Scholar]
- Mathew A., Harris A. M., Marshall M. J., Ross G. W. The use of analytical isoelectric focusing for detection and identification of beta-lactamases. J Gen Microbiol. 1975 May;88(1):169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- Matsubara N., Yotsuji A., Kumano K., Inoue M., Mitsuhashi S. Purification and some properties of a cephalosporinase from Proteus vulgaris. Antimicrob Agents Chemother. 1981 Jan;19(1):185–187. doi: 10.1128/aac.19.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Emergence of resistance during therapy with the newer beta-lactam antibiotics: role of inducible beta-lactamases and implications for the future. Rev Infect Dis. 1983 Jul-Aug;5(4):639–648. doi: 10.1093/clinids/5.4.639. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Type I beta-lactamases of gram-negative bacteria: interactions with beta-lactam antibiotics. J Infect Dis. 1986 Nov;154(5):792–800. doi: 10.1093/infdis/154.5.792. [DOI] [PubMed] [Google Scholar]
- Sawai T., Kanno M., Tsukamoto K. Characterization of eight beta-lactamases of Gram-negative bacteria. J Bacteriol. 1982 Nov;152(2):567–571. doi: 10.1128/jb.152.2.567-571.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Tsukamoto K. Cefoxitin, N-formimidoyl thienamycin, clavulanic acid, and penicillanic acid sulfone as suicide inhibitors for different types of beta-lactamases produced by gram-negative bacteria. J Antibiot (Tokyo) 1982 Nov;35(11):1594–1602. doi: 10.7164/antibiotics.35.1594. [DOI] [PubMed] [Google Scholar]
- Sawai T., Yoshida T., Tsukamoto K., Yamagishi S. A set of bacterial strains for evaluation of beta-lactamase-stability of beta-lactam antibiotics. J Antibiot (Tokyo) 1981 Oct;34(10):1318–1326. doi: 10.7164/antibiotics.34.1318. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Matthew M. The beta-lactamases of gram-negative bacteria and their role in resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1976 Jun;2(2):115–157. doi: 10.1093/jac/2.2.115. [DOI] [PubMed] [Google Scholar]
- Vu H., Nikaido H. Role of beta-lactam hydrolysis in the mechanism of resistance of a beta-lactamase-constitutive Enterobacter cloacae strain to expanded-spectrum beta-lactams. Antimicrob Agents Chemother. 1985 Mar;27(3):393–398. doi: 10.1128/aac.27.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waley S. G. A spectrophotometric assay of beta-lactamase action on penicillins. Biochem J. 1974 Jun;139(3):789–790. doi: 10.1042/bj1390789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann B. Genetic and biochemical basis of resistance of Enterobacteriaceae to beta-lactam antibiotics. J Antimicrob Chemother. 1986 Oct;18 (Suppl B):31–38. doi: 10.1093/jac/18.supplement_b.31. [DOI] [PubMed] [Google Scholar]
- Yang Y. J., Livermore D. M., Williams R. J. Chromosomal beta-lactamase expression and antibiotic resistance in Enterobacter cloacae. J Med Microbiol. 1988 Mar;25(3):227–233. doi: 10.1099/00222615-25-3-227. [DOI] [PubMed] [Google Scholar]



