Abstract
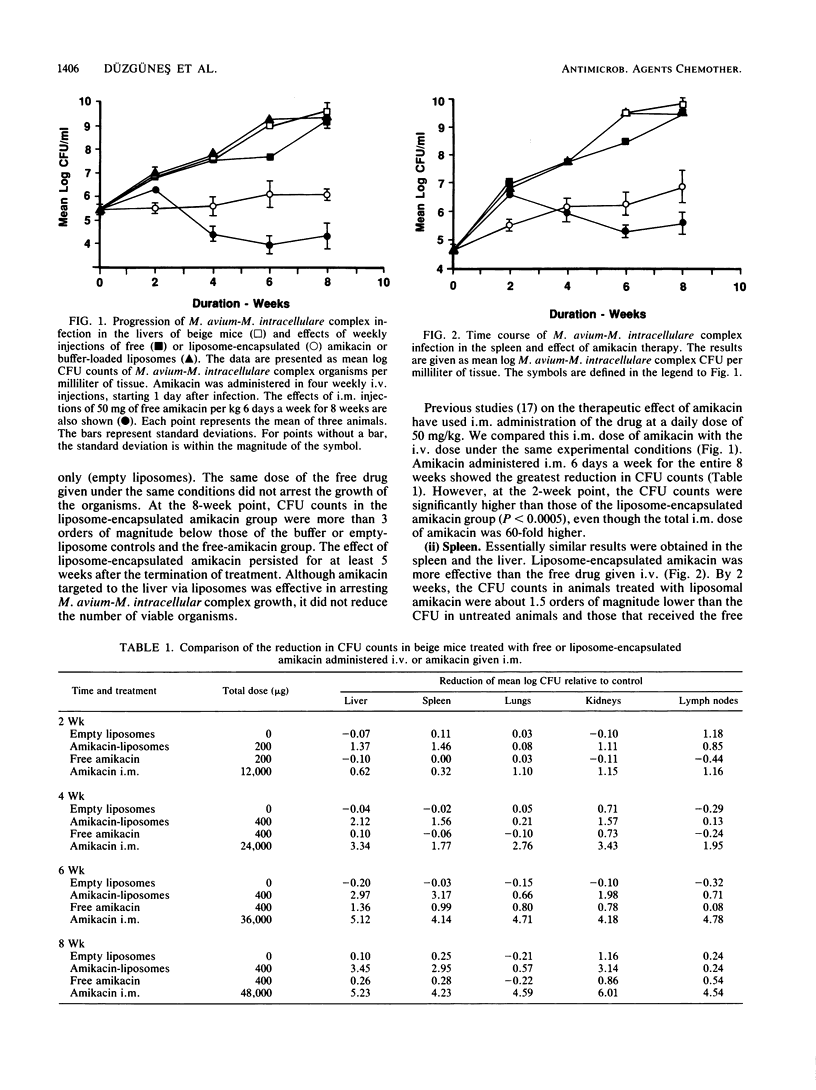
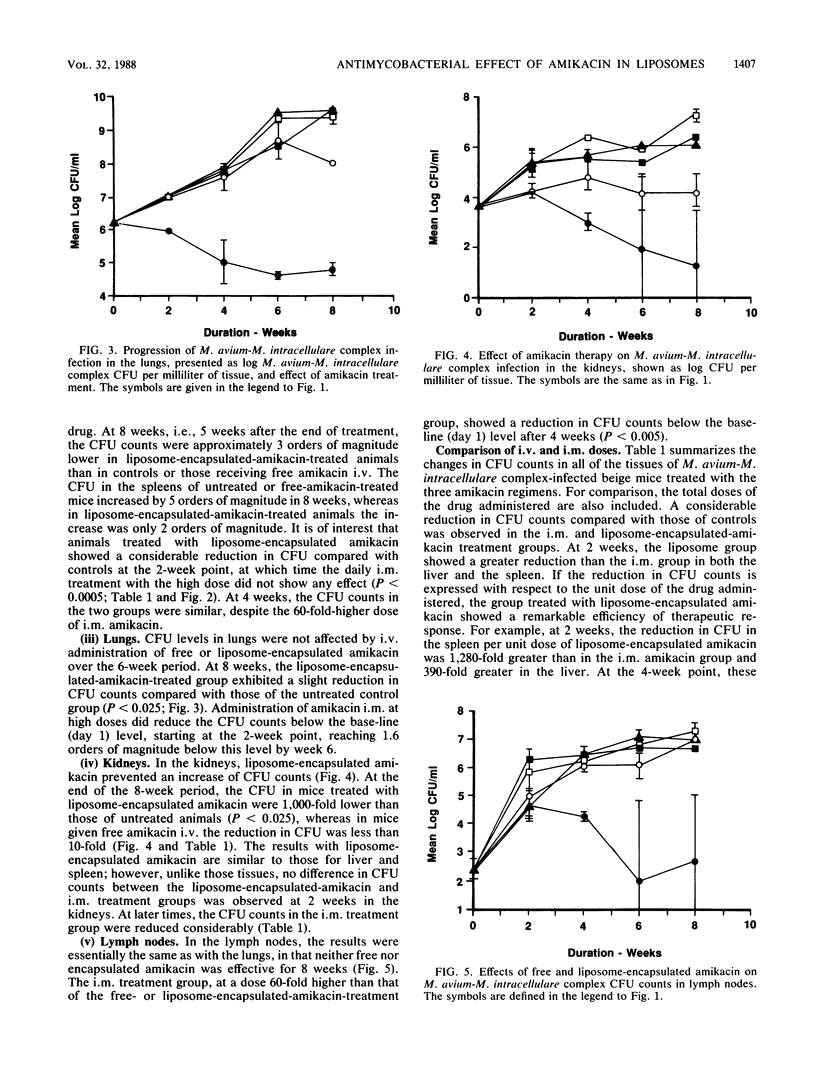
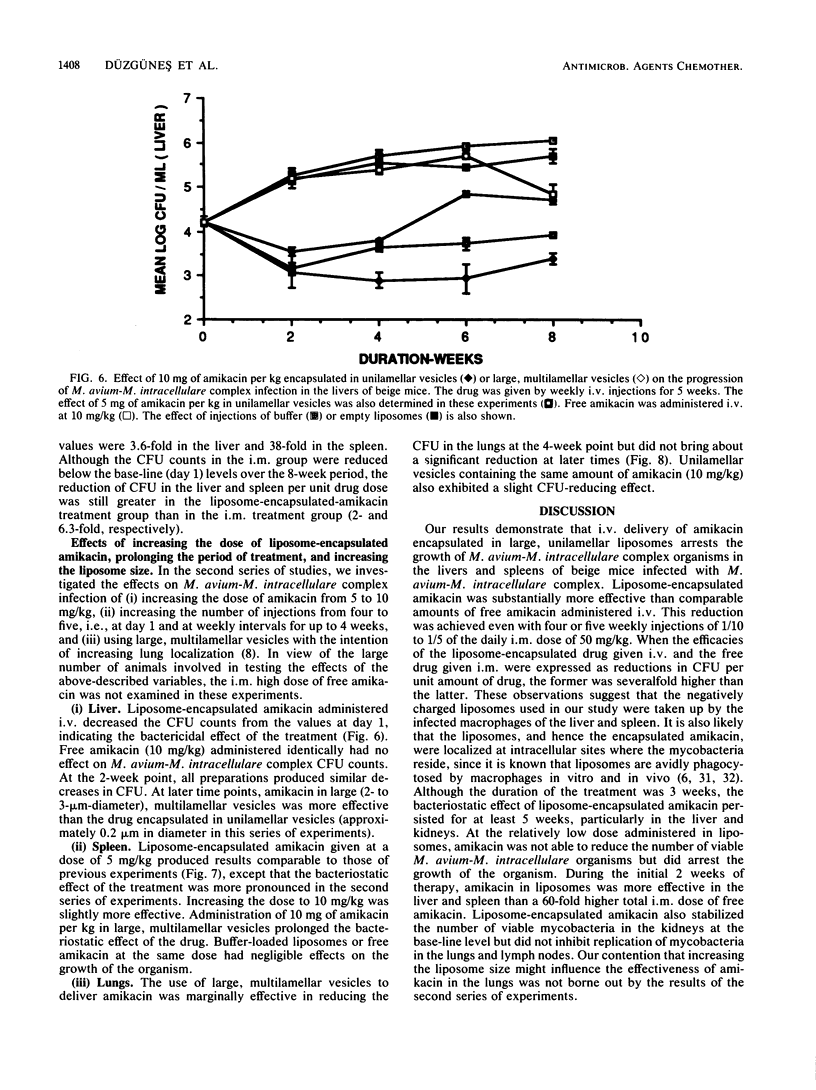
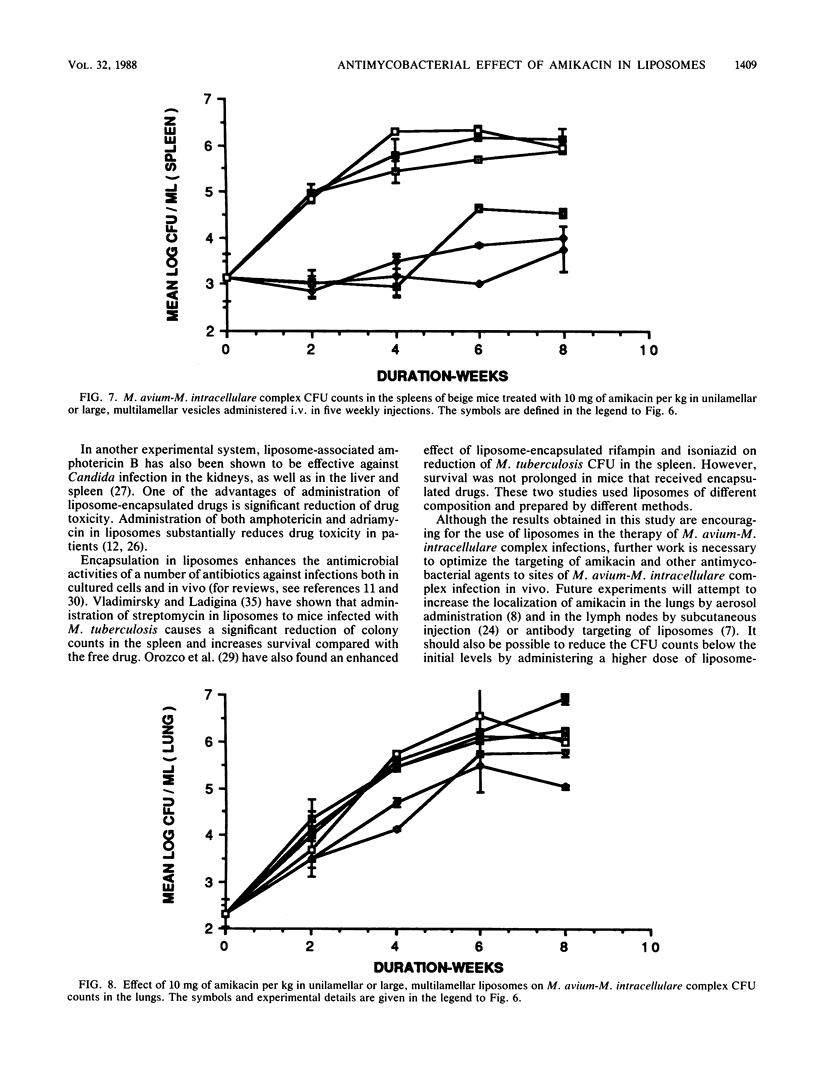
We examined the therapeutic effects of free and liposome-encapsulated amikacin on Mycobacterium avium-M. intracellulare complex infection by using the beige-mouse model of the disease. In the first series of studies, intravenous administration of four weekly doses of 5 mg of amikacin per kg encapsulated in large (approximately 0.4-micron diameter), unilamellar liposomes arrested the growth of M. avium-M. intracellulare complex organisms in the liver, as measured by CFU counts. M. avium-M. intracellulare complex levels in untreated animals and in those treated with the same dose of free amikacin increased by several orders of magnitude over 8 weeks. Liposome-encapsulated amikacin was also effective against M. avium-M. intracellulare complex organisms in the spleen and kidneys, reducing the CFU counts by about 1,000-fold compared with those of both untreated controls and free-drug-treated mice. In the lungs, a slight reduction in CFU was observed in the liposome-encapsulated-amikacin-treated group, but only at the 8-week point. Neither free nor liposome-encapsulated amikacin reduced the colony counts in the lymph nodes compared with those of control animals. Reductions in CFU in all organs greater than those caused by the liposome preparation could be achieved by intramuscular administration of free amikacin, but only at a 10-fold-higher dose given 6 days a week for 8 weeks. In the second series of studies, we investigated the effects of (i) doubling the dose of liposome-encapsulated amikacin and (ii) increasing the size of the liposomes and prolonging the treatment to five injections. Administration of 10 mg of amikacin per kg in liposomes 2 to 3 micrometer in diameter was more effective in the liver than 5 or 10 mg of amikacin per kg in liposomes 0.2 micrometer in diameter. A slight reduction in the CFU levels in the lungs was observed with the higher dose, irrespective of liposome size. Our results indicate that liposome-based delivery of amikacin enhances its anti-M. intracellulare complex activity, particularly in the liver, spleen, and kidney, and may therefore improve the therapy of this disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alving C. R., Steck E. A., Chapman W. L., Jr, Waits V. B., Hendricks L. D., Swartz G. M., Jr, Hanson W. L. Therapy of leishmaniasis: superior efficacies of liposome-encapsulated drugs. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2959–2963. doi: 10.1073/pnas.75.6.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D., Gold J. W., Dryjanski J., Whimbey E., Polsky B., Hawkins C., Brown A. E., Bernard E., Kiehn T. E. Treatment of infections in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1985 Nov;103(5):738–743. doi: 10.7326/0003-4819-103-5-738. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bakker-Woudenberg I. A., Lokerse A. F., Roerdink F. H., Regts D., Michel M. F. Free versus liposome-entrapped ampicillin in treatment of infection due to Listeria monocytogenes in normal and athymic (nude) mice. J Infect Dis. 1985 May;151(5):917–924. doi: 10.1093/infdis/151.5.917. [DOI] [PubMed] [Google Scholar]
- Baron E. J., Young L. S. Amikacin, ethambutol, and rifampin for treatment of disseminated Mycobacterium avium-intracellulare infections in patients with acquired immune deficiency syndrome. Diagn Microbiol Infect Dis. 1986 Sep;5(3):215–220. doi: 10.1016/0732-8893(86)90004-0. [DOI] [PubMed] [Google Scholar]
- Daemen T., Veninga A., Roerdink F. H., Scherphof G. L. In vitro activation of rat liver macrophages to tumoricidal activity by free or liposome-encapsulated muramyl dipeptide. Cancer Res. 1986 Sep;46(9):4330–4335. [PubMed] [Google Scholar]
- Debs R. J., Heath T. D., Papahadjopoulos D. Targeting of anti-Thy 1.1 monoclonal antibody conjugated liposomes in Thy 1.1 mice after intravenous administration. Biochim Biophys Acta. 1987 Jul 23;901(2):183–190. doi: 10.1016/0005-2736(87)90114-3. [DOI] [PubMed] [Google Scholar]
- Debs R. J., Straubinger R. M., Brunette E. N., Lin J. M., Lin E. J., Montgomery A. B., Friend D. S., Papahadjopoulos D. P. Selective enhancement of pentamidine uptake in the lung by aerosolization and delivery in liposomes. Am Rev Respir Dis. 1987 Mar;135(3):731–737. doi: 10.1164/arrd.1987.135.3.731. [DOI] [PubMed] [Google Scholar]
- Dijkstra J., van Galen W. J., Hulstaert C. E., Kalicharan D., Roerdink F. H., Scherphof G. L. Interaction of liposomes with Kupffer cells in vitro. Exp Cell Res. 1984 Jan;150(1):161–176. doi: 10.1016/0014-4827(84)90711-0. [DOI] [PubMed] [Google Scholar]
- Düzgüneş N., Wilschut J., Hong K., Fraley R., Perry C., Friend D. S., James T. L., Papahadjopoulos D. Physicochemical characterization of large unilamellar phospholipid vesicles prepared by reverse-phase evaporation. Biochim Biophys Acta. 1983 Jul 13;732(1):289–299. doi: 10.1016/0005-2736(83)90214-6. [DOI] [PubMed] [Google Scholar]
- Emmen F., Storm G. Liposomes in treatment of infectious diseases. Pharm Weekbl Sci. 1987 Jun 19;9(3):162–171. doi: 10.1007/BF01967536. [DOI] [PubMed] [Google Scholar]
- Gangadharam P. R., Edwards C. K., 3rd, Murthy P. S., Pratt P. F. An acute infection model for Mycobacterium intracellulare disease using beige mice: preliminary results. Am Rev Respir Dis. 1983 May;127(5):648–649. doi: 10.1164/arrd.1983.127.5.648. [DOI] [PubMed] [Google Scholar]
- Gangadharam P. R., Edwards C. K., 3rd Release of superoxide anion from resident and activated mouse peritoneal macrophages infected with Mycobacterium intracellulare. Am Rev Respir Dis. 1984 Nov;130(5):834–838. doi: 10.1164/arrd.1984.130.5.834. [DOI] [PubMed] [Google Scholar]
- Gangadharam P. R., Kesavalu L., Rao P. N., Perumal V. K., Iseman M. D. Activity of amikacin against Mycobacterium avium complex under simulated in vivo conditions. Antimicrob Agents Chemother. 1988 Jun;32(6):886–889. doi: 10.1128/aac.32.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadharam P. R., Perumal V. K., Jairam B. T., Rao P. N., Nguyen A. K., Farhi D. C., Iseman M. D. Activity of rifabutin alone or in combination with clofazimine or ethambutol or both against acute and chronic experimental Mycobacterium intracellulare infections. Am Rev Respir Dis. 1987 Aug;136(2):329–333. doi: 10.1164/ajrccm/136.2.329. [DOI] [PubMed] [Google Scholar]
- Gangadharam P. R., Perumal V. K., Podapati N. R., Kesavalu L., Iseman M. D. In vivo activity of amikacin alone or in combination with clofazimine or rifabutin or both against acute experimental Mycobacterium avium complex infections in beige mice. Antimicrob Agents Chemother. 1988 Sep;32(9):1400–1403. doi: 10.1128/aac.32.9.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadharam P. R., Pratt P. F. In vitro response of murine alveolar and peritoneal macrophages to Mycobacterium intracellulare. Am Rev Respir Dis. 1983 Dec;128(6):1044–1047. doi: 10.1164/arrd.1983.128.6.1044. [DOI] [PubMed] [Google Scholar]
- Greene J. B., Sidhu G. S., Lewin S., Levine J. F., Masur H., Simberkoff M. S., Nicholas P., Good R. C., Zolla-Pazner S. B., Pollock A. A. Mycobacterium avium-intracellulare: a cause of disseminated life-threatening infection in homosexuals and drug abusers. Ann Intern Med. 1982 Oct;97(4):539–546. doi: 10.7326/0003-4819-97-4-539. [DOI] [PubMed] [Google Scholar]
- Hawkins C. C., Gold J. W., Whimbey E., Kiehn T. E., Brannon P., Cammarata R., Brown A. E., Armstrong D. Mycobacterium avium complex infections in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1986 Aug;105(2):184–188. doi: 10.7326/0003-4819-105-2-184. [DOI] [PubMed] [Google Scholar]
- Heifets L. B., Iseman M. D., Lindholm-Levy P. J. Determination of MICs of conventional and experimental drugs in liquid medium by the radiometric method against Mycobacterium avium complex. Drugs Exp Clin Res. 1987;13(9):529–538. [PubMed] [Google Scholar]
- Horsburgh C. R., Jr, Mason U. G., 3rd, Farhi D. C., Iseman M. D. Disseminated infection with Mycobacterium avium-intracellulare. A report of 13 cases and a review of the literature. Medicine (Baltimore) 1985 Jan;64(1):36–48. doi: 10.1097/00005792-198501000-00003. [DOI] [PubMed] [Google Scholar]
- Iseman M. D., Corpe R. F., O'Brien R. J., Rosenzwieg D. Y., Wolinsky E. Disease due to Mycobacterium avium-intracellulare. Chest. 1985 Feb;87(2 Suppl):139S–149S. doi: 10.1378/chest.87.2.139s. [DOI] [PubMed] [Google Scholar]
- Kaledin V. I., Matienko N. A., Nikolin V. P., Gruntenko Y. V., Budker V. G., Vakhrusheva T. E. Subcutaneously injected radiolabeled liposomes: transport to the lymph nodes in mice. J Natl Cancer Inst. 1982 Jul;69(1):67–71. [PubMed] [Google Scholar]
- Kende M., Alving C. R., Rill W. L., Swartz G. M., Jr, Canonico P. G. Enhanced efficacy of liposome-encapsulated ribavirin against Rift Valley fever virus infection in mice. Antimicrob Agents Chemother. 1985 Jun;27(6):903–907. doi: 10.1128/aac.27.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Berestein G., Bodey G. P., Frankel L. S., Mehta K. Treatment of hepatosplenic candidiasis with liposomal-amphotericin B. J Clin Oncol. 1987 Feb;5(2):310–317. doi: 10.1200/JCO.1987.5.2.310. [DOI] [PubMed] [Google Scholar]
- Lopez-Berestein G., Mehta R., Hopfer R. L., Mills K., Kasi L., Mehta K., Fainstein V., Luna M., Hersh E. M., Juliano R. Treatment and prophylaxis of disseminated infection due to Candida albicans in mice with liposome-encapsulated amphotericin B. J Infect Dis. 1983 May;147(5):939–945. doi: 10.1093/infdis/147.5.939. [DOI] [PubMed] [Google Scholar]
- Masur H., Tuazon C., Gill V., Grimes G., Baird B., Fauci A. S., Lane H. C. Effect of combined clofazimine and ansamycin therapy on Mycobacterium avium-Mycobacterium intracellulare bacteremia in patients with AIDS. J Infect Dis. 1987 Jan;155(1):127–129. doi: 10.1093/infdis/155.1.127. [DOI] [PubMed] [Google Scholar]
- Orozco L. C., Quintana F. O., Beltrán R. M., de Moreno I., Wasserman M., Rodriguez G. The use of rifampicin and isoniazid entrapped in liposomes for the treatment of Murine tuberculosis. Tubercle. 1986 Jun;67(2):91–97. doi: 10.1016/0041-3879(86)90002-4. [DOI] [PubMed] [Google Scholar]
- Poste G., Bucana C., Raz A., Bugelski P., Kirsh R., Fidler I. J. Analysis of the fate of systemically administered liposomes and implications for their use in drug delivery. Cancer Res. 1982 Apr;42(4):1412–1422. [PubMed] [Google Scholar]
- Spanjer H. H., Morselt H., Scherphof G. L. Lactosylceramide-induced stimulation of liposome uptake by Kupffer cells in vivo. Biochim Biophys Acta. 1984 Jul 11;774(1):49–55. doi: 10.1016/0005-2736(84)90273-6. [DOI] [PubMed] [Google Scholar]
- Szoka F., Olson F., Heath T., Vail W., Mayhew E., Papahadjopoulos D. Preparation of unilamellar liposomes of intermediate size (0.1-0.2 mumol) by a combination of reverse phase evaporation and extrusion through polycarbonate membranes. Biochim Biophys Acta. 1980 Oct 2;601(3):559–571. doi: 10.1016/0005-2736(80)90558-1. [DOI] [PubMed] [Google Scholar]
- Vladimirsky M. A., Ladigina G. A. Antibacterial activity of liposome-entrapped streptomycin in mice infected with mycobacterium tuberculosis. Biomed Pharmacother. 1982;36(8-9):375–377. [PubMed] [Google Scholar]
- Wong B., Edwards F. F., Kiehn T. E., Whimbey E., Donnelly H., Bernard E. M., Gold J. W., Armstrong D. Continuous high-grade mycobacterium avium-intracellulare bacteremia in patients with the acquired immune deficiency syndrome. Am J Med. 1985 Jan;78(1):35–40. doi: 10.1016/0002-9343(85)90458-9. [DOI] [PubMed] [Google Scholar]
- Yajko D. M., Nassos P. S., Hadley W. K. Therapeutic implications of inhibition versus killing of Mycobacterium avium complex by antimicrobial agents. Antimicrob Agents Chemother. 1987 Jan;31(1):117–120. doi: 10.1128/aac.31.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Inderlied C. B., Berlin O. G., Gottlieb M. S. Mycobacterial infections in AIDS patients, with an emphasis on the Mycobacterium avium complex. Rev Infect Dis. 1986 Nov-Dec;8(6):1024–1033. doi: 10.1093/clinids/8.6.1024. [DOI] [PubMed] [Google Scholar]
- Young L. S. Management of opportunistic infections complicating the acquired immunodeficiency syndrome. Med Clin North Am. 1986 May;70(3):677–692. doi: 10.1016/s0025-7125(16)30946-4. [DOI] [PubMed] [Google Scholar]
- Zakowski P., Fligiel S., Berlin G. W., Johnson L., Jr Disseminated Mycobacterium avium-intracellulare infection in homosexual men dying of acquired immunodeficiency. JAMA. 1982 Dec 10;248(22):2980–2982. doi: 10.1001/jama.1982.03330220024029. [DOI] [PubMed] [Google Scholar]



