Abstract
Mammalian cerebral cortex is composed of a multitude of different areas that are each specialized for a unique purpose. It is unclear whether the activity pattern and modality of sensory inputs to cortex play an important role in the development of cortical regionalization. The modality of sensory inputs to cerebral cortex can be altered experimentally. Neonatal diversion of retinal axons to the auditory thalamus (cross-modal rewiring) results in a primary auditory cortex (AI) that resembles the primary visual cortex in its visual response properties and topography. Functional reorganization could occur because the visual inputs use existing circuitry in AI, or because the early visual inputs promote changes in AI’s circuitry that make it capable of constructing visual receptive field properties. The present study begins to distinguish between these possibilities by exploring whether the callosal connectivity of AI is altered by early visual experience. Here we show that early visual inputs to auditory thalamus can reorganize callosal connections in auditory cortex, causing both a reduction in their extent and a reorganization of the pattern. This result is distinctly different from that in deafened animals, which have widespread callosal connections, as in early postnatal development. Thus, profound changes in cortical circuitry can result simply from a change in the modality of afferent input. Similar changes may underlie cortical compensatory processes in deaf and blind humans.
The development of the unique properties of each sensory cortical area (cortical regionalization) can be influenced by activity-dependent factors (1, 2). Clinical evidence supporting this idea includes the observation that in blind and deaf patients, the “unused” cortical area can be taken over and used by a different sensory modality (3–5). Cross-modal plasticity can also be obtained in animal models (6–10), but the underlying mechanisms are unknown. In the cross-modal plasticity paradigm employed here, primary auditory cortex (AI) receives visual information during postnatal development. Because the manipulation is done peripherally, the modality and thus the pattern of input activity are changed without damaging the thalamocortical pathway carrying the information to cortex (11, 12). Under these conditions, AI exhibits receptive field properties usually seen in visual cortex, such as a retinotopic map and orientation-selective visual neurons (13, 14). A major question that remains is whether the anomalous visual inputs make use of existing circuitry in AI, or whether they cause active changes in AI’s circuitry, organizing it appropriately for processing visual information.
Callosal connections unite functionally and/or topographically related cells in the two cortical hemispheres, and they are specific and different in each sensory cortical area (15–19). The projection is exuberant early in postnatal development (20, 21), and becomes progressively restricted under the influence of sensory experience (16). Callosal connectivity patterns thus provide an ideal model for answering the question posed above. We show here that changing the modality of sensory inputs alters the pattern of callosal connections seen in normal AI, suggesting that the visual inputs actively remodel cortical circuitry for a new purpose.
Preliminary results from some of these experiments have been published previously (22).
EXPERIMENTAL PROCEDURES
Animals.
Callosal connectivity was examined in three groups of adult ferrets (Mustela putorius furo); one with visual inputs rerouted to the medial geniculate nucleus (MGN) at birth (“cross-modal,” n = 16), one with bilateral cochlear ablation (“deaf,” n = 4), and a group of normal ferrets (n = 6). Timed pregnant jills were obtained from Marshall Farms (North Rose, NY), and their kits were used for the cross-modal and deaf groups. Normal animals were obtained as adults. All procedures involving live animals were approved by an institutional animal care and use committee, and they strictly followed U.S. Department of Agriculture and National Institutes of Health guidelines for humane animal care and use.
Cross-Modal Rerouting Surgery.
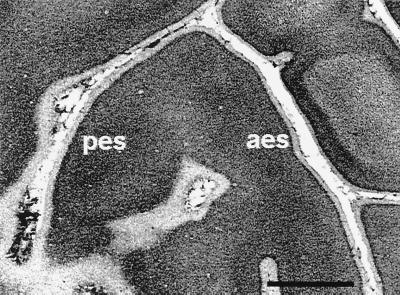
Within 24 hr of birth, kits were anesthetized with isoflurane and prepared for surgery. Under sterile conditions, the brain was exposed and the superficial layers of the left superior colliculus (SC), left primary visual cortex (V1), and both inferior colliculi (IC) were cauterized. In many cases there was some damage to the right SC as well. After the incision had been closed, the kit was allowed to recover from anesthesia and then returned to the ferret colony. The cortical lesions resulted in some disturbance to the sulcal pattern of AI, but the anterior and posterior ectosylvian sulci as well as the pseudosylvian sulcus were always present. Furthermore, there was no visible alteration of the laminar cytoarchitecture of AI (Fig. 1). Under these conditions, retinal axons project to the contralateral medial geniculate nucleus (MGN) and to a lesser extent to the ipsilateral MGN (11, 23–26).
Figure 1.
Photomicrograph of a Nissl-stained, flattened section through AI in a cross-modal ferret (the same animal as Fig. 3C). Most of this tangential 50-μm section cuts through layer 2/3, although layer 4 can be seen in the center. pes and aes, posterior and anterior ectosylvian sulci. Despite some changes in the sulcal pattern resulting from the visual cortical lesion, the cytoarchitecture looks undisturbed. (Scale bar = 2.0 mm.)
Cochlear Ablation.
Cochlear ablations were performed on postnatal day 14 ferret kits, 2 weeks before the onset of auditory function (27). Kits were anaesthetized with isoflurane and both cochleae were surgically destroyed as described previously (28, 29). After the incision had been closed, the kit was allowed to recover from anesthesia and was then returned to the colony.
Before callosal injections (see below), auditory brainstem responses (ABRs) were recorded to confirm functional deafness. Under ketamine (30 mg/kg)/xylazine (1 mg/kg) anesthesia, pin electrodes (Astro-Med, West Warwick, RI) were inserted subcutaneously over the vertex and the mastoid of each ear, and the hind leg (ground). A Bio-Logic Express ABR system was used to present 100-μs rarefaction clicks at a rate of 27.7 per sec. Stimuli were presented by means of Etymotic ER-3 insert earphones (Etymotic Research, Elk Grove Village, IL). Responses were bandpass filtered (30–1500 Hz) and differentially amplified with a gain of 150,000. At least 1500 presentations were averaged. Maximum stimulus intensity was 103.5 dB peak sound pressure level sound pressure level. All of the normal animals tested and none of the deafened animals used in this study exhibited a response to the clicks.
Further confirmation of cochlear ablations was obtained through postmortem inspection of the temporal bones, and in most cases, through histological examination of the temporal bones and brainstems as described previously (28, 29). Significant brainstem degeneration occurs after deafening in early infancy and, in this study, provided supportive data for the temporal bone histology. In all cases, the cochleae appeared to be fully destroyed.
Callosal Injections.
For callosal labeling experiments, adult ferrets were given preoperative injections of atropine (0.04 mg/kg), doxapram (2 mg/kg), and dexamethasone (40 mg/kg) and were then preanesthetized with ketamine (40 mg/kg) and xylazine (1–2 mg/kg), and intubated for deep anesthesia with isoflurane. Body temperature was maintained with a heating pad, the ECG was continuously monitored, and fluids were supplied intravenously. The head was placed in a stereotaxic device and the left (normal and deaf animals) or right (cross-modal animals) primary auditory cortex (AI) was exposed. A series of 15–25 50-μl injections of 2% wheat germ-agglutinated and 30% free horseradish peroxidase in sterile saline was made throughout the extent of the AI with a Hamilton syringe to which had been attached a broken micropipette (≈100-μm diameter at the tip) marked at 1-mm intervals along its length. The location of AI was defined by the location of sulci (12, 30–32). The incision was closed and the ferret was injected with amoxicillin (30 mg/kg), allowed to recover from the anesthetic, and given supportive care and analgesics until the terminal procedure. The number of injections did not affect the extent of label. The success of this method in completely filling the corpus callosum was supported by our finding that the ventral nucleus of the ipsilateral MGN was labeled in its entirety in each case.
Eye Injections.
In two representative animals, tracer injections were made to delineate the retino-MGN projections. Injections of 8 μl of cholera toxin (2% in water; List Biological Laboratories, Campbell, CA) were made into the posterior chamber of each eye after the callosal injections. Cholera toxin produced consistent terminal label in the targets of the retinal axons, including the MGN in the cross-modal animals.
Histology.
Three to five days after the callosal injections, ferrets were deeply anesthetized and perfused lightly with buffered 2% paraformaldehyde. The hemispheres were flattened and the tissue was postfixed in buffered 4% paraformaldehyde. After cryoprotection in 30% sucrose, the tissue was sectioned in the tangential (cortex) or coronal plane (thalamus and midbrain) at 50 μm. One series of sections at 200-μm intervals was stained with cresyl violet to reveal cytoarchitecture. An adjacent series of sections was treated with a modified tetramethylbenzidine histochemical procedure for visualization of horseradish peroxidase (33, 34). The sections that went through layer 2/3, which is the main interhemispherically projecting layer, were chosen for photography, three-dimensional reconstruction, and quantitative analysis. Note that in the micrographs presented, the left AIs have been photographically reversed so that all appear from the same perspective. Visualization of retinal terminals in the MGN after injection of cholera toxin in the eyes was made possible by an anti-cholera toxin antibody (List) and a fluorescent secondary antibody [FITC (Vector) or Cy-2 (Jackson ImmunoResearch) -conjugated rabbit anti-goat IgG].
Lesion Reconstruction.
Reconstruction of lesions in the cross-modal animals was done by tracing cresyl violet-stained sections through the superior and inferior colliculi and thalamus at 200-μm intervals, measuring the remaining extent of the colliculi and lateral geniculate nucleus bilaterally and in three dimensions, and comparing it to the size of these structures in normal adults. Lesions were grouped according to the amount of colliculus present [the colliculi’s absence contributes more to rerouting than any loss of thalamus (26)]. Lesions were categorized as small (less than 50% of the colliculi absent), medium (50–75% of the colliculi absent), and large (more than 75% of the colliculi absent).
Quantitative Data Analysis.
We measured the three-dimensional size and shape of all dense patches of callosal terminal label as well as the three largest patches of label in layer 2/3 for each treatment group, using Neurolucida reconstruction software (MicroBrightField, Colchester, VT). Patch boundaries were defined as where the terminal label density reached near confluence, using the same criteria for all cases, by a third-party observer blind to the experimental condition of the animals. In the absence of coronal sections to determine the cytoarchitectural boundaries of AI (35), it was defined as the surface of the ectosylvian gyrus inside the anterior and posterior ectosylvian sulci and medial to the pseudosylvian sulcus (30–32). The area lateral to AI is the secondary auditory cortical area, AII (32, 36, 37), and we thus drew the AI/AII boundary at the apex of the pseudosylvian sulcus. Previous studies have confirmed that AI remains on the crown of the ectosylvian gyrus despite the cross-modal rewiring lesions (11–14). Patches in many cases extended into AII, and we included these patches in all analyses except as noted. The length of the major and minor axis and the lateral extent of each patch was calculated by MicroBrightField’s Neuromorph software. Area, not volume, was the size measure used because the lateral extent of the projections, not depth within layer 2/3, was the measure of interest. In addition, the proportion of AI occupied by the patches was calculated. The means of each group were compared by using an unpaired t test, and standard errors are presented for each comparison.
RESULTS
Multiple injections of tracer throughout the extent of primary auditory cortex produced patches of anterograde and retrograde label in the MGN and the contralateral AI. The resulting pattern of callosal label was analyzed both qualitatively and quantitatively.
Callosal Projection Patterns in Normal Auditory Cortex.
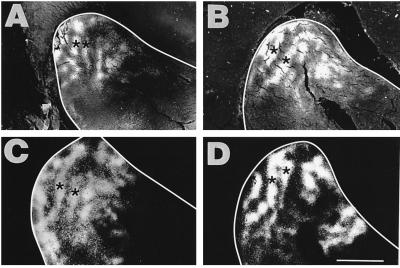
A total of six normal ferrets were used. We found that the form of callosal projections in normal adult ferrets (Fig. 2) resembled that in cats (15, 38), although the patches of label in the ferrets had sharper boundaries than reported in cats. The most striking observation was the presence of 2–3 bands of callosal terminals and cells in AI (asterisks in Fig. 2) and extending into AII. These bands ran in a mainly medial-to-lateral direction within the caudal arm of the ectosylvian gyrus, parallel to the tonotopic map in ferrets (see Discussion), and were 300–500 μm wide in upper layer 2/3. They were not visible in the deeper layers 5 and 6. These bands probably correspond to binaural bands seen in cats (15, 39). In addition to the bands there were small patches of label located along the ectosylvian and pseudosylvian sulci, and within the anterior arm of the ectosylvian gyrus extending into AII. As we reported previously (32), the callosally projecting cells were mainly in layer 2/3 (Fig. 2 A, B, and D). However, there was also a substantial but sparser and more diffuse projection from layer 5, as seen in cats (40). The projections were largely reciprocal, although somata were somewhat more widely distributed than terminals (Fig. 2C).
Figure 2.
Pattern of callosal connections in the right AI of four normal animals after multiple injections of retro- and anterograde tracer in the contralateral AI. The ectosylvian sulcus is outlined in white in this and the other micrographs. A, B, and D are slightly more superficial sections than C, illustrating that the labeled somata in layer 2/3, which are at a deeper level than the terminals, were somewhat less restricted in their distribution than the anterogradely labeled terminals. Asterisks show the location of the two clearest stripes or bands of callosal label observed in normal animals. (Scale bar = 2.5 mm.)
Callosal Projection Patterns in Auditory Cortex of Cross-Modal Ferrets.
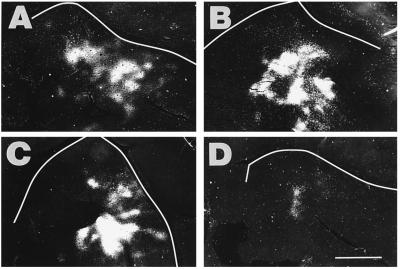
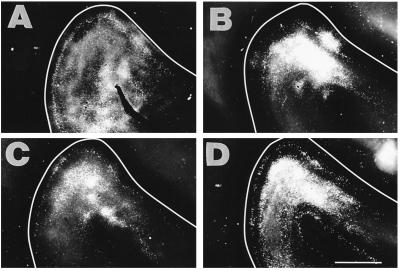
Retinal projections into the auditory thalamus were induced from the day of birth. Sixteen of these “cross-modal” ferrets were used for tracer injections in the right AI, contralateral to the visual cortical lesions. Six had large lesions (more than 75% of the midbrain inferior and superior colliculi absent), six had medium lesions (50–75% of the midbrain absent), and four had small lesions (less than 50% missing). Micrographs from four of the animals that had large lesions are presented here in order of increasing lesion size in Fig. 3, and micrographs from four animals with medium lesions are presented in order of decreasing lesion size in Fig. 4.
Figure 3.
Pattern of callosal connections in the left AI from cross-modal animals with large rerouting lesions. The panels are arranged from the smallest (IA) to the largest (D) lesion in this large lesion size class. Note that the orientation of the left AI in the micrographs of cross-modal ferret cortices (Figs. 3 and 4) has been photographically reversed from left to right to facilitate comparisons with deaf and normal animals. Tracer was injected throughout the right AI, and it revealed an absence of bands and a concentration of the label in the ventral part of the left AI. The extent of labeling was markedly reduced compared with normal animals, especially in D. (Scale bar = 2.5 mm.)
Figure 4.
Pattern of callosal connections in the left AI from cross-modal animals with smaller rerouting lesions. The panels are arranged from largest (A) to smallest (D) lesion in this medium-sized lesion class, in the opposite order from Fig. 3. Tracer was injected in the right AI. A and B are from animals with larger lesions than C and D. The alterations in the callosal patterns were less severe with smaller lesions. (Scale bar = 2.5 mm.)
In the cross-modal ferrets with medium and large lesions, the left AI (opposite the injections) contained patches of callosal label rather than bands (Figs. 3 and 4). There was a conspicuous reduction in the number of labeled callosal somata and terminals overall, and a particular decline in the caudomedial portion of AI, where the bands would normally be located. The extent of the alteration depended on the size of the early rerouting lesion. With larger lesions, and presumably increased retinal input to the MGN, the caudomedial portion of the ectosylvian gyrus was more likely to be devoid of label (Fig. 3 and Fig. 4 A and B), although strong callosal connections persisted in the ventral part of AI and into AII in all but the animal with the largest lesion (Fig. 3D). In animals with medium-sized lesions (Fig. 4), the pattern looked less abnormal, and in the case of the smallest lesion shown (Fig. 4D) there was some suggestion of bands. The callosal projection patterns in animals with small lesions were affected only minimally and are not shown.
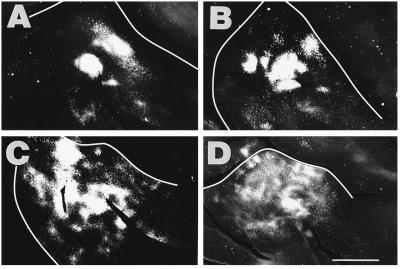
Callosal Projection Patterns in Auditory Cortex of Deaf Ferrets.
Four deafened ferrets were used to examine the effects of an absence of normal auditory input on callosal connectivity in AI. All were functionally deafened as demonstrated by one or more methods, including auditory brainstem recordings, cochlear and brainstem histology, and informal behavioral assessments. These various methods provide a high level of confidence in the effectiveness of the deafening procedure.
The callosal labeling pattern in the deaf ferrets was much more diffuse than in the other groups of animals (Fig. 5), although there was some trace of a banding pattern in a similar location and orientation to that seen in normal animals in one deaf animal (Fig. 5A), and a small number of patches along the edge of the sulci in all deaf animals (Fig. 5 A–C). Deaf animals overall had fewer and larger patches than the other groups (see below).
Figure 5.
Pattern of callosal connections in early-deafened animals. The somata and terminals were more widespread than in normal or cross-modal animals. In the AI shown in A, some evidence of a banded distribution of terminals was evident, and in all of the animals there were a few patches of terminal label at the edge of the sulcus, but these were less common than in the other groups. (Scale bar = 2.5 mm.)
Deafening in infancy produces a loss of neurons in the cochlear nucleus (29, 41) and superior olivary complex (28, 42) and changes in the projections between the cochlear nucleus and the inferior colliculus (28, 43). We therefore anticipated that the thalamocortical projection between MGN and AI might also be affected by cochlear ablation, although this has not yet been examined. We did observe that the extent and intensity of labeling in the MGN were similar to those in normal animals, demonstrating that the connection between MGN and AI remained intact.
Quantitative Analysis of Callosal Projection Patterns.
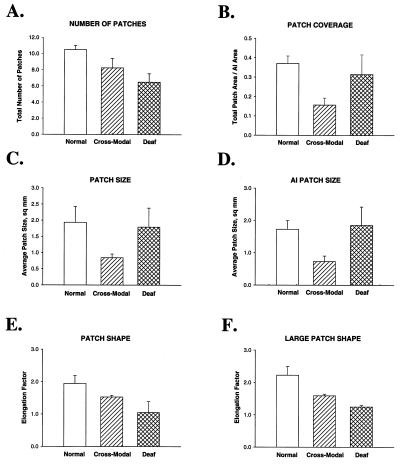
Totals of four normal, eight cross-modal, and four deaf animals were used for further, quantitative analysis of callosal projection patterns. As expected from our qualitative observations, we found that the number of patches of terminal label in the auditory cortical area differed among the three groups (Fig. 6A), with normal animals having the greatest number and deaf animals the least number of patches. The mean number of patches in the cross-modal animals was intermediate between the other two groups. There was a significant difference in the number of patches between the normal and the deaf groups (P = 0.01). Because of the sparseness and wide distribution of the label in the deaf animals, terminals often were not clustered enough to be defined as a patch by the observer. In contrast, even though the patches were largely restricted to lateral AI in the cross-modal cases, the number of patches was not significantly smaller than normal (P = 0.22).
Figure 6.
Quantitative analysis of the data. (A) Comparison of the number of patches of callosal terminal label on the ectosylvian gyrus in the three different groups. The deaf animals had significantly fewer patches. (B) Proportion of AI occupied by patches of callosal terminal label (coverage area). The patches occupied the smallest proportion of AI in the cross-modal animals. (C) Comparison of the size of patches of callosal label in AI and AII considered together. Mean patch size (area) is plotted for each group. Cross-modal animals had significantly smaller patches than the other two groups. (D) Comparison of the patches located within the estimated borders of AI for each group. Again the patches were smallest in the cross-modal animals. (E) Comparison of the shape of patches of callosal label, with respect to elongation along the major axis. A mean ratio of major to minor axis length was calculated in each case for all patches within the ectosylvian gyrus. There was a significant difference in patch elongation only between the normal and cross-modal groups. (F) Comparison of the shape of the three largest patches of callosal label revealed significant differences with respect to all comparisons.
After noting that the callosal projections in cross-modal animals were preferentially found in the lateral portion of AI, whereas the projections in normal and deaf animals were more evenly distributed, the proportion of AI occupied by patches of terminal label was determined. This coverage factor was highly significantly different (P = 0.004) between the normal and the cross-modal animals (Fig. 6B). There was a nonsignificant (P = 0.10) trend for the coverage in deaf animals to be greater than that in cross-modal animals, but the coverage in normal and deaf animals was similar (P = 0.62).
We measured the size and shape of all patches of terminal label within the ectosylvian gyrus, which includes AI and AII. The reason for this was that many of the patches cross the AI/AII boundary, and furthermore, the location of the boundary cannot be determined precisely from tangential sections. The mean size of the patches in the cross-modal animals was significantly smaller than in the normal animals (P = 0.01) or in the deaf animals (P = 0.05), but patch size was not significantly different between the normal and deaf groups (P = 0.86) (Fig. 6C). The relationship was similar when the patches within the estimated boundaries of AI were measured separately (Fig. 6D) (normal vs. cross-modal P = 0.009; normal vs. deaf P = 0.85; deaf vs. cross-modal P = 0.03).
The shape of the patches was quantified by calculating their major axis/minor axis ratio (elongation factor). When all patches were compared, there were no significant differences between normal and deaf groups or cross-modal and deaf groups in the mean elongation ratio (P = 0.08 for normal vs. deaf and P = 0.07 for cross-modal vs. deaf), but the difference between normal and cross-modal groups did reach significance (P = 0.05) (Fig. 6E). To relate the pattern of callosal label to the bands in normal animals, we next restricted the analysis to the three largest patches, which included the two or three elongated bands in each of the normal animals (Fig. 6F). In this analysis, the mean elongation in normal animals was significantly greater than in either the cross-modal group (P = 0.008) or the deaf group (P = 0.01). There was also a highly significant difference in patch shape between the deaf and cross-modal groups (P = 0.001).
Thus, the patches of callosal terminal label in the cross-modal animals were significantly smaller and less elongated than in the normal animals, and smaller than in the deaf animals, whereas the patches of callosal label in the deaf animals were larger than in cross-modal animals but fewer in number and less elongated than in either normal or cross-modal animals. These quantitative results support our qualitative observation that loss of sensory input and change of input modality each produce different and specific alterations of callosal circuitry.
DISCUSSION
We have shown here that changing the modality of sensory inputs to auditory cortex, without changing the identity of the thalamic fibers carrying the new information, causes a reorganization of callosal circuitry. We found that, after rerouting of visual input to the auditory thalamus, the banded pattern of callosal connections normally seen is converted into a series of smaller patches restricted to lateral AI. This pattern was distinct from a deprivation effect, as deafened animals had diffuse, widespread, and unbanded callosal connections in AI. These results strongly suggest that the visual inputs cause an active remodeling of cortical circuitry, and they argue against the idea that visual inputs in our cross-modal rewiring paradigm are making use only of existing connections in AI.
Callosal Connectivity Patterns in Normal Animals.
The pattern of callosal connections in normal ferrets resembled the binaural bands in cats (15) except that the orientation of the bands was roughly perpendicular to that in cats (see ref. 39). However, the tonotopic map in ferrets is also oriented roughly opposite to that in cats (30, 31). Thus the callosal bands are oriented as would be expected if they serve the same binaural processing function in ferrets and cats. A study using a different injection method described a similar pattern of callosal connections in normal ferret AI (36).
Callosal Connectivity Patterns in Deaf Animals.
The distribution of callosal connections in ferrets deafened by cochlear ablation was more widespread and uniform than normal, and it resembled the pattern of callosal connections in early postnatal cats (21). It is possible that a lack of appropriate sensory input freezes AI’s callosal connectivity pattern in an early, activity-independent developmental stage. Alternatively, the connections may expand postnatally from a more restricted state.
The expansion of callosal connections in deafened animals is particularly interesting because of the variable effects of deafferentation in other sensory cortical areas. In cat visual cortex, bilateral deprivation or enucleation result in a reduced callosal projection (44–46), whereas monocular manipulations result in expanded callosal projections (16, 47, 48). However, binocularity seems not to play an important organizing role in this pathway, since strabismus does not affect the pattern or number of callosal cells (49, 50). Rather, the outcome of deafferentation is predicted by the effect of the manipulation on retinotopic correspondence between the two hemispheres (18, 19, 44, 49, 50). In deaf animals, there may be no information available on which to base elimination of connections, as in the retention of overlapped corticocortical (51) or thalamocortical (52) arbors after visual deprivation. If the auditory afferents are the only, or the main, source of organizing information for callosal connections in AI, then deafening might be expected to preserve the initial widespread condition. Thus, these results support the contention that sensory experience is important in the organization and regionalization of sensory neocortex.
Callosal Connectivity Patterns in Cross-Modal Animals.
Providing ferret AI with visual input from early in postnatal development can produce an AI that resembles primary visual cortex very closely in its topography and response properties (13, 14). However, the gross pattern of AI’s connectivity with other auditory structures remains unaltered, and in particular the thalamocortical pathway remains elongated along the isofrequency axis (12, 32). Therefore, it seems likely that the visual response properties observed in AI of cross-modally rewired ferrets arise from activity-dependent changes in AI’s intrinsic circuitry. Alternatively, AI and visual cortex may be sufficiently similar in their intrinsic circuitry that they can process each other’s inputs without modification. For example, primary sensory cortex could serve simply as a contrast enhancer, employing standard lateral inhibitory circuits. Our results address this question by demonstrating the importance of patterned thalamic activity in specifying the callosal connectivity aspect of cortical identity. We are currently exploring whether the cross-modal rewiring procedure can influence other aspects of auditory cortical circuitry, and our results suggest that both horizontal connectivity and the organization of inhibitory circuits are affected by visual activity (53–56).
Methodological Considerations.
In this study, tracer injections in cross-modal ferrets were made only in the right AI, contralateral to the neonatal superior colliculus and visual cortex lesions. This protocol allowed us to analyze the distribution of axonal terminations from cells located throughout the AI on the injected (right) side into the AI on the left side, and also the pattern of somata backfilled by injections throughout AI on the injected side. In a previous study of cross-modal ferrets, we made smaller injections within AI of the lesioned hemisphere (32), but the complete tangential pattern of callosal injections was not revealed by that method. It is possible that the distribution of somata and terminals in the right AI would be different than reported here for the left side. This possibility seems unlikely at least in animals with the largest lesions in which there was significant bilateral damage to the inferior and superior colliculi. It would be of interest to test this in the future and also to examine the pattern of callosal projections in animals with intentionally bilateral cross-modal rerouting surgery.
The cochlear ablation procedure was done at 2 weeks of age, and the cross-modal surgery was done on the day of birth. Postponing the cochlear ablations greatly increases survival of kits, but postponing the rerouting surgery reduces its effect. In ferret kits, the ears become functional (27) and the eyes open at approximately 1 month of age. External sensory input was thus prevented in both modalities. Moreover, cochlear ablation at postnatal day 14 produces neuron loss in the cochlear nucleus and superior olivary complex equivalent to that obtained after ablation at earlier ages (ref. 29; D.R.M. and S.L.P., unpublished observation).
Relation to Previous Studies.
Neocortex begins as a relatively uniform sheet of progenitor epithelial cells, which progressively expands and subdivides into numerous morphologically and functionally distinct areas. Previous studies regarding the role of sensory inputs in this regionalization are in disagreement over the relative roles of extrinsic and intrinsic factors (see ref. 2 for review). Our experiments support the hypothesis that the pattern of activity in the thalamocortical inputs during development is a source of cortical patterning information at the level of cortical circuit formation and possibly for cortical regionalization in general.
Further investigation is planned to determine the function of the callosal projections in the cross-modal animals. The patches may be connecting neurons with similar visual or auditory response properties. We did not find that the pattern of callosal connections in the cross-modal AI resembled that in normal primary visual cortex, where only the neurons representing the visual midline have callosal connections. In fact, a previous mapping study in cross-modal ferrets places the vertical meridian of the visual field in the medial portion of AI (13), the portion that this study shows to be specifically devoid of callosal connections. The right AI in our cross-modal animals probably represents auditory information, especially in animals with smaller unilateral lesions, and the callosal pathway may provide this auditory information to the left AI combined with its visual input from the ipsilateral MGN. The pattern of callosal connections may reflect a compromise in representing visual and auditory information on one cortical surface. What form would this compromise take? Because visual information is mapped in two dimensions, azimuth and elevation, whereas auditory topography is one-dimensional, representing the frequency map of the cochlea, it seems unlikely that both modalities could be represented in individual cells, or even in the same portion of AI. The two modalities could instead be arranged as in the tectum, with alignment between representations of auditory and visual space (57–59). However, it seems more likely, based on the work of Constantine-Paton and colleagues (60), that the two maps would parse the cortical territory by Hebbian mechanisms, leading to fractured, segregated maps of visual and auditory information. The absence of callosal projections from the medial AI in several of the animals with large lesions is consistent with the hypothesis that callosal projections between neurons receiving input from different modalities are eliminated to prevent perceptual confusion. Supporting this idea are our recent data showing that the pattern of horizontally projecting axons within AI is complementary to the callosal pattern in cross-modal ferrets, extending specifically into the medial area that is devoid of callosal connections (53, 55, 56).
The present data suggest that the cross-modal plasticity observed in deaf and blind humans, where the preserved modality exhibits heightened sensitivity and invades the cortical area normally subserving the missing modality (3, 5), may result from the ability of sensory afferents to reorganize their cortical targets. Such a process opens up the possibility of manipulating the afferent pathways for clinical purposes.
Acknowledgments
We thank Julie Harral and Astou Coly for technical help, Charles Henley for help with brainstem recordings, Eric Knudsen and Terry Sejnowski for valuable discussions, and Jaime Olavarria and Paul Katz for helpful criticisms of the manuscript. This work was supported by grants from the National Science Foundation (IBN-95-11430), the Whitehall Foundation (F93-28), the Fight for Sight Research Division of Prevent Blindness America, and the Georgia Research Alliance to S.L.P., and the Medical Research Council (U.K.) to D.R.M.
ABBREVIATIONS
- AI
primary auditory cortex
- AII
secondary auditory cortex
- MGN
medial geniculate nucleus
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Shatz C J. Science. 1992;258:237–238. doi: 10.1126/science.1357747. [DOI] [PubMed] [Google Scholar]
- 2.Levitt P, Barbe M F, Eagleson K L. Annu Rev Neurosci. 1997;20:1–24. doi: 10.1146/annurev.neuro.20.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Neville H J, Schmidt A, Kutas M. Brain Res. 1983;266:127–132. doi: 10.1016/0006-8993(83)91314-8. [DOI] [PubMed] [Google Scholar]
- 4.Neville H J. Ann N Y Acad Sci. 1990;608:71–87. doi: 10.1111/j.1749-6632.1990.tb48892.x. [DOI] [PubMed] [Google Scholar]
- 5.Cohen L G, Celnik P, Pascual-Leone A, Cornwell B, Faiz L, Dambrosias J, Honda M, Sadato N, Gerloff C, Catala M D, Hallett M. Nature (London) 1997;389:180–186. doi: 10.1038/38278. [DOI] [PubMed] [Google Scholar]
- 6.Schneider G E. Brain Behav Evol. 1973;8:73–109. doi: 10.1159/000124348. [DOI] [PubMed] [Google Scholar]
- 7.Rebillard G, Carlier E, Rebillard M, Pujol R. Brain Res. 1977;129:162–164. doi: 10.1016/0006-8993(77)90980-5. [DOI] [PubMed] [Google Scholar]
- 8.Heil P, Bronchti G, Wollberg Z, Scheich H. NeuroReport. 1991;2:735–738. doi: 10.1097/00001756-199112000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Rauschecker J P. Trends Neurosci. 1995;18:36–43. doi: 10.1016/0166-2236(95)93948-w. [DOI] [PubMed] [Google Scholar]
- 10.Rauschecker J P. Prog Brain Res. 1996;112:313–323. doi: 10.1016/s0079-6123(08)63338-5. [DOI] [PubMed] [Google Scholar]
- 11.Sur M, Garraghty P E, Roe A W. Science. 1988;242:1437–1441. doi: 10.1126/science.2462279. [DOI] [PubMed] [Google Scholar]
- 12.Pallas S L, Roe A W, Sur M. J Comp Neurol. 1990;298:50–68. doi: 10.1002/cne.902980105. [DOI] [PubMed] [Google Scholar]
- 13.Roe A W, Pallas S L, Hahm J, Sur M. Science. 1990;250:818–820. doi: 10.1126/science.2237432. [DOI] [PubMed] [Google Scholar]
- 14.Roe A W, Pallas S L, Kwon Y, Sur M. J Neurosci. 1992;12:3651–3664. doi: 10.1523/JNEUROSCI.12-09-03651.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imig T J, Brugge J F. J Comp Neurol. 1978;182:637–660. doi: 10.1002/cne.901820406. [DOI] [PubMed] [Google Scholar]
- 16.Innocenti G M, Frost D O. Nature (London) 1979;280:231–234. doi: 10.1038/280231a0. [DOI] [PubMed] [Google Scholar]
- 17.Segraves M A, Rosenquist A C. J Neurosci. 1982;2:1090–1107. doi: 10.1523/JNEUROSCI.02-08-01090.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olavarria J F, Van Sluyters R C. Anat Embryol (Berlin) 1995;191:239–242. doi: 10.1007/BF00187822. [DOI] [PubMed] [Google Scholar]
- 19.Lewis J W, Olavarria J F. J Comp Neurol. 1995;361:119–137. doi: 10.1002/cne.903610110. [DOI] [PubMed] [Google Scholar]
- 20.Innocenti G M, Fiore L, Caminiti R. Neurosci Lett. 1977;4:237–242. doi: 10.1016/0304-3940(77)90185-9. [DOI] [PubMed] [Google Scholar]
- 21.Feng J Z, Brugge J F. J Comp Neurol. 1983;214:416–426. [Google Scholar]
- 22.Pallas S L, Wright J. Soc Neurosci Abstr. 1993;19:675. [Google Scholar]
- 23.Pallas S L, Hahm J, Sur M. J Comp Neurol. 1994;347:1–20. doi: 10.1002/cne.903490303. [DOI] [PubMed] [Google Scholar]
- 24.Pallas S L, Sur M. J Comp Neurol. 1994;347:1–14. doi: 10.1002/cne.903490304. [DOI] [PubMed] [Google Scholar]
- 25.Angelucci A, Clascá F, Bricolo E, Cramer K S, Sur M. J Neurosci. 1997;17:2040–2055. doi: 10.1523/JNEUROSCI.17-06-02040.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angelucci A, Clascá F, Sur M. J Comp Neurol. 1998;400:417–439. doi: 10.1002/(sici)1096-9861(19981026)400:3<417::aid-cne10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 27.Moore D R, Hine J E. Dev Brain Res. 1992;66:229–235. doi: 10.1016/0165-3806(92)90084-a. [DOI] [PubMed] [Google Scholar]
- 28.Moore D R, Kowalchuk N E. J Comp Neurol. 1988;272:503–515. doi: 10.1002/cne.902720405. [DOI] [PubMed] [Google Scholar]
- 29.Moore D R. J Comp Neurol. 1990;302:810–823. doi: 10.1002/cne.903020412. [DOI] [PubMed] [Google Scholar]
- 30.Kelly J B, Judge P W, Phillips D P. Hearing Res. 1986;24:111–115. doi: 10.1016/0378-5955(86)90054-7. [DOI] [PubMed] [Google Scholar]
- 31.Phillips D P, Judge P W, Kelly J B. Brain Res. 1988;443:281–294. doi: 10.1016/0006-8993(88)91622-8. [DOI] [PubMed] [Google Scholar]
- 32.Pallas S L, Sur M. J Comp Neurol. 1993;337:317–333. doi: 10.1002/cne.903370212. [DOI] [PubMed] [Google Scholar]
- 33.Mesulam M M. J Neurophysiol. 1978;26:106–117. doi: 10.1177/26.2.24068. [DOI] [PubMed] [Google Scholar]
- 34.Gibson A R, Hansma D I, Houk J C, Robinson F R. Brain Res. 1984;298:235–241. doi: 10.1016/0006-8993(84)91423-9. [DOI] [PubMed] [Google Scholar]
- 35.Rose J E. J Comp Neurol. 1949;91:408–440. doi: 10.1002/cne.900910305. [DOI] [PubMed] [Google Scholar]
- 36.Wallace M N, Harper M S. Exp Brain Res. 1997;116:367–374. doi: 10.1007/pl00005764. [DOI] [PubMed] [Google Scholar]
- 37.Wallace M N, Roeda D, Harper M S. Exp Brain Res. 1997;117:488–500. doi: 10.1007/s002210050245. [DOI] [PubMed] [Google Scholar]
- 38.Imig T J, Reale R A, Brugge J F, Morel A, Adrian H O. Two Hemispheres-One Brain: Functions of the Corpus Callosum. New York: Liss; 1986. pp. 103–115. [Google Scholar]
- 39.Kelly J B, Judge P W. J Neurophysiol. 1994;71:904–913. doi: 10.1152/jn.1994.71.3.904. [DOI] [PubMed] [Google Scholar]
- 40.Winguth S D, Winer J A. J Comp Neurol. 1986;248:36–56. doi: 10.1002/cne.902480104. [DOI] [PubMed] [Google Scholar]
- 41.Tierney T S, Russell F A, Moore D R. J Comp Neurol. 1997;378:295–306. doi: 10.1002/(sici)1096-9861(19970210)378:2<295::aid-cne11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 42.Moore D R. NeuroReport. 1992;3:269–272. doi: 10.1097/00001756-199203000-00014. [DOI] [PubMed] [Google Scholar]
- 43.Moore D R. J Comp Neurol. 1994;339:301–310. doi: 10.1002/cne.903390209. [DOI] [PubMed] [Google Scholar]
- 44.Innocenti G M, Frost D O. Exp Brain Res. 1980;39:365–375. doi: 10.1007/BF00239301. [DOI] [PubMed] [Google Scholar]
- 45.Innocenti G M, Frost D O, Illes J. J Neurosci. 1985;5:255–267. doi: 10.1523/JNEUROSCI.05-02-00255.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frost D O, Moy Y P. Exp Brain Res. 1989;78:203–213. doi: 10.1007/BF00230700. [DOI] [PubMed] [Google Scholar]
- 47.Lund R D, Mitchell D E, Henry G H. Brain Res. 1978;144:169–172. doi: 10.1016/0006-8993(78)90445-6. [DOI] [PubMed] [Google Scholar]
- 48.Berman N E, Payne B R. Brain Res. 1983;274:201–212. doi: 10.1016/0006-8993(83)90697-2. [DOI] [PubMed] [Google Scholar]
- 49.Olavarria J F. J Comp Neurol. 1996;366:643–655. doi: 10.1002/(SICI)1096-9861(19960318)366:4<643::AID-CNE6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 50.Bourdet C, Olavarria J F, Van Sluyters R C. J Comp Neurol. 1996;366:259–269. doi: 10.1002/(SICI)1096-9861(19960304)366:2<259::AID-CNE6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 51.Callaway E M, Katz L C. Proc Natl Acad Sci USA. 1991;88:745–749. doi: 10.1073/pnas.88.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antonini A, Stryker M P. J Neurosci. 1993;13:3549–3573. doi: 10.1523/JNEUROSCI.13-08-03549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao, W.-J. & Pallas, S. L. J. Neurosci., in press.
- 54.Pallas S L, Booth V, Cynader M. Soc Neurosci Abstr. 1994;20:875. [Google Scholar]
- 55.Pallas S L, Gao W-J. Invest Ophthalmol Visual Sci. 1999;40:S645. [Google Scholar]
- 56.Gao, W.-J., Moore, D. R. & Pallas, S. L. (1999) Soc. Neurosci. Abstr. 25, in press.
- 57.Knudsen E I. J Neurosci. 1982;2:1177–1194. doi: 10.1523/JNEUROSCI.02-09-01177.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.King A J. Exp Physiol. 1993;78:559–590. doi: 10.1113/expphysiol.1993.sp003708. [DOI] [PubMed] [Google Scholar]
- 59.King A J, Carlile S. Hearing Res. 1994;81:137–149. doi: 10.1016/0378-5955(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 60.Constantine-Paton M. Trends Neurosci. 1983;6:32–36. [Google Scholar]