Abstract
Nitric oxide (NO) produced by the endothelium diffuses both into the lumen and to the smooth muscle cells according to the concentration gradient in each direction. The extremely high reaction rate between NO and hemoglobin (Hb), kHb= 3–5 × 107 M−1⋅s−1, suggests that most of the NO produced would be consumed by Hb in the red blood cells (RBCs), which then would block the biological effect of NO. Therefore, specific mechanisms must exist under physiological conditions to reduce the NO consumption by RBCs, in which the Hb concentration is very high (24 mM heme). By using isolated microvessels as a bioassay, here we show that physiological concentrations of RBCs in the presence of intravascular flow does not inhibit NO-mediated vessel dilation, suggesting that RBCs under this condition are not an NO scavenger. On the other hand, RBCs (50% hematocrit) without intravascular flow reduce NO-mediated dilation to serotonin by 30%. In contrast, free Hb (10 μM) completely inhibits NO-mediated dilation with or without intravascular flow. The effect of flow on NO consumption by RBCs may be attributed to the formation of an RBC-free zone near the vessel wall, which is caused by hydrodynamic forces on particles. Intravascular flow does not affect the reaction rate between NO and free Hb in the lumen, because the latter forms a homogeneous solution and is not subject to the hydrodynamic separation. However, intravascular flow only partially contributes to the reduced consumption of NO by RBCs, because without the flow, the NO consumption by RBCs is already about 3 orders of magnitude slower than free Hb.
Nitric oxide (NO) is a biological messenger that participates in neurotransmission, vascular regulation, and immunological responses. It is produced in many cell types such as neurons, endothelial cells vascular smooth muscle cells, and macrophages. Since the discovery of its biological activity, the roles of NO in physiological and pathophysiological processes have been seen as increasingly important. In the cardiovascular system, NO has been documented to participate in the regulation of vascular tone and permeability (1, 2), platelet adhesion and aggregation (3, 4), smooth muscle proliferation (5, 6), and endothelial cell-leukocyte interactions (1, 7). The functions of NO in vivo have been clearly demonstrated by administration of l-arginine analogs that block NO production. For example, the administration of NG-monomethyl-l-arginine (l-NMMA) has been shown to cause hypertension in vivo (8) and abolish shear-induced, NO-mediated vessel dilation both in vivo (9) and in isolated intact vessel preparation (10).
Despite the well-documented importance of NO, the transfer of NO from the producing cell to the target is poorly understood, because NO, as a free radical, can be degraded in a variety of reactions. In particular, NO reacts with deoxy- and oxy-hemoglobin (Hb) at a very high rate to form nitrosyl-Hb (HbNO), and metHb, respectively. The bimolecular reaction rate constants for these reactions are on the order of 3–5 × 107 M−1⋅s−1 (11, 12), which gives a half-life of about 1 μsec for NO in blood according to the direct interpretation of the in vitro data. With this short half-life, the concentration of NO would be too low to activate its target enzyme, soluble guanylate cyclase. Indeed, modeling analyses have shown that if oxyHb in the red blood cell (RBC) reacts with NO as fast as free Hb does, then the NO concentrations in vascular smooth muscle will be too low to activate soluble guanylate cyclase (13, 14), the main target enzyme for NO.
Several lines of in vivo and in vitro evidence suggest that Hb is an effective NO scavenger that depletes NO. For example, infusion of a free Hb solution into experimental animals or human subjects results in hypertension (15), most likely because of the oxidative reaction of NO with oxyHb in the circulation. Furthermore, 3–6 μM of free Hb can abolish NO-mediated vasodilation in vitro (16, 17). Given these results, it is unclear how NO exerts any regulatory function in vivo, where the normal Hb concentration in the blood is about 12–15 mM (heme basis), almost 1,000 times higher than that required for free Hb to abolish the NO effect.
Several theories have been proposed to explain this paradox. In particular, the formation of S-nitrosyl Hb (18, 19) has been suggested as a mechanism to package and transport NO. Independent of this theory, external diffusion limitation has been implicated in reducing the rate of NO consumption by RBCs (14, 20, 21). The external limitation may be caused by either an undisturbed (boundary) layer surrounding each RBC (20) or an RBC-free zone near the vessel wall (14, 21). Liu et al. (20) have shown that the NO consumption by a dilute RBC suspension is much slower than that of a free Hb solution with an equivalent Hb concentration. This observation was attributed to the undisturbed layer surrounding each RBC. Vaughn et al. (14) used a diffusion and reaction model to predict that the existence of an RBC-free zone would increase the NO concentration in the smooth muscle layer by 2- to 3-fold. Whether such an increase is physiologically significant remains to be determined. Butler et al. (21) also modeled the NO diffusion and reaction system in a vessel with an RBC-free zone. Under their assumptions, the model shows that the existence of the RBC-free zone allows the outflow of NO into the smooth muscle layer. Therefore, intravascular flow may affect the NO consumption rate by RBCs. Intuitively, it can be argued that intravascular flow would reduce the thickness of the undisturbed layer and thus increase NO consumption by RBC. On the other hand, intravascular flow also generates an RBC-free zone near the vessel wall and thus reduces the interaction between the endothelial released NO and RBC. Therefore, the net effect of flow on NO consumption by RBCs is difficult to predict. By using isolated microvessels as a bioassay, we investigated the effect of intravascular flow on NO consumption by RBCs under physiological levels of hematocrit.
MATERIALS AND METHODS
General Preparation.
Pigs (12 weeks old of either sex) were sedated with intramuscular injection of telazol (4.4 mg/kg) and xylazine (2.2 mg/kg), then anesthetized and heparinized with an i.v. injection with pentobarbital sodium (20 mg/kg) and heparin (1,000 units/kg), respectively, via the marginal ear vein. Pigs were intubated and ventilated with room air. After a left thoracotomy was performed, the heart was electrically fibrillated, excised, and immediately placed in cold (5°C) saline solution, and the blood was collected for RBC isolation.
Isolation and Cannulation of Microvessels.
The techniques for identification and isolation of coronary microvessels have been described (22, 23). In brief, a mixture of India ink and gelatin in physiological salt solution (PSS) containing 145.0 mM NaCl, 4.7 mM KCl, 2.0 mM CaCl2, 1.17 mM MgSO4, 1.2 mM NaH2PO4, 5.0 mM glucose, 2.0 mM pyruvate, 0.02 mM EDTA, and 3.0 mM 3-(N-morpholino) propanesulfonic acid (Mops) buffer was perfused under a low pressure (20 cm H2O) into the left anterior descending artery (0.5 ml) and the circumflex artery (0.5 ml) to enable visualization of the coronary microvessels. Subepicardial arteriolar branches (50–100 μm internal diameter and 0.6–1.0 mm in length without branches) from the left anterior descending or circumflex arteries were selected and carefully dissected from the surrounding cardiac tissue under cold (5°C) PSS containing 1% of BSA (Amersham Pharmacia) at pH 7.4. Each isolated arteriole then was transferred for cannulation to a Lucite vessel chamber containing PSS-albumin equilibrated with room air at ambient temperature. One end of the microvessel was cannulated with a glass micropipette (40 μm in tip diameter) filled with filtered PSS-albumin, and the outside of the microvessel was securely tied to the pipette with 11-O ophthalmic suture (Alcon Laboratories, Fort Worth, TX). The ink-gelatin solution inside the vessel was flushed out at a low perfusion pressure (<20 cm H2O). Then, the other end of the vessel was cannulated with a second micropipette and tied with suture. We previously have shown that the ink-gelatin solution has no detectable detrimental effect on either endothelial or vascular smooth muscle function. (22, 23). Electrical resistances (measured by LCR Bridge Circuit, model LCR-740, Leader Electronics, Yokohama, Japan) of the micropipettes were matched (± 0.5%).
Instrumentation.
After cannulation of a blood vessel, the chamber was transferred to the stage of an inverted microscope (model IM35, Zeiss) coupled to a charge-coupled device camera (KP-161, Hitachi), video micrometer (Microcirculation Research Institute, Texas A&M University Health Science Center), and video recorder (JVC BR-S600U). The micropipettes were connected to independent reservoir systems, and intravascular pressures were measured through sidearms of the two reservoir lines by low-volume displacement strain-gauge transducers (Statham P23 Db, Gould, Cleveland). The vessel was set to its in situ length and allowed to develop a spontaneous tone at 60 cm H2O luminal pressure without flow. This pressure corresponds to that found in arterioles of similar sizes in the beating heart (24). After the vessel developed basal tone, the experimental interventions were performed and internal diameters of the vessel were measured throughout the experiment by using video microscope with a data acquisition system (25).
RBC Isolation and OxyHb Preparation.
The RBCs were isolated by the method of Beutler (26). Briefly, a column was prepared by pouring a 1:1 (dry weight) mixture of α-cellulose and microcrystalline cellulose (Sigmacell type 50, Sigma) mixed with PSS into the barrel of a 10-ml syringe. The syringe was filled to the 4-ml mark and washed with 5 ml of PSS. Two milliliters of blood was allowed to flow through the column and washed through with PSS. The cell suspensions from several columns were diluted about 10× by ice-cold PSS and centrifuged at 850 g for 15 min. This washing procedure was repeated once. The packed erythrocytes were suspended in 1× volume of PSS. Before use, the erythrocytes were washed again to remove any free Hb caused by possible lysis. During the purification and washing processes, the RBC was almost completely oxygenated, as confirmed spectrophotometrically.
Porcine Hb was prepared from lyophilized powder (Sigma) by the method of Di Iorio (27). Briefly, Hb was dissolved in PSS to prepare a stock solution of approximately 1 mM. To assure that the Hb was in the ferrous form, the Hb solution was cooled to 4°C, reduced with sodium dithionite, and immediately desalted by passing through a column of Sephadex G-25 (Amersham Pharmacia) that had been previously equilibrated with 4°C PSS. The concentration of the resulting Hb was assayed by spectrophotometer. The Hb was refrigerated and used within 2 days. Before the experiment, the Hb was oxygenated with 95% O2 for 5 min.
Effect of Hb and RBCs on NO-Mediated Coronary Arteriolar Dilation.
We previously have demonstrated that coronary arteriolar dilation in response to flow (shear) (25) and serotonin (28) stimulation is mediated by the NO released from the endothelium. To study the effect of Hb and RBCs on NO-mediated flow-induced vasodilation, the vascular response to a vehicle flow driven by a pressure gradient (ΔP = 4, 10, 20, 40, and 60 cm H2O) across the length of the vessel initially was examined. The luminal flow was produced by simultaneously moving the reservoirs in opposite directions of the same magnitude. We have demonstrated that the luminal flow is increased linearly with increasing ΔP and the range of mean volumetric flows for ΔP between 0 and 60 cm H2O was 0–34.8 nl/sec. The effect of Hb or RBCs on flow-induced vasodilation was examined by perfusing the vessels with PSS containing various concentrations of Hb or RBCs. To study the effect of Hb and RBCs on serotonin-induced dilation, the vessels were pressurized without flow by setting both reservoirs to the same hydrostatic level. Serotonin (10−10 M to 10−6 M) was administered cumulatively into the vessel bath, and the concentration-dependent dilation was examined in the absence (vehicle control) and presence of luminal RBCs or Hb. Chemicals and drugs were obtained from Sigma, except when stated otherwise.
Data Analysis.
At the end of each experiment, the vessel was relaxed with sodium nitroprusside (10−4 M) to obtain its maximal diameter at 60 cm H2O intraluminal pressure (28). All diameter changes were normalized to the maximal diameter and expressed as a percentage of maximal dilation. All data are presented as mean ± SEM. Statistical comparisons of dose- or flow-dependent vasomotor responses under various treatments were performed with one- or two-way ANOVA when appropriate and tested with Fisher’s protected least significant difference multiple range test. Differences in resting diameter in response to serotonin (10−7 M) before and after Hb or RBC treatments were compared by the paired Student’s t test. Significance was accepted at P < 0.05.
RESULTS
Intravascular Flow Does Not Affect the NO Consumption Rate by Free Hb.
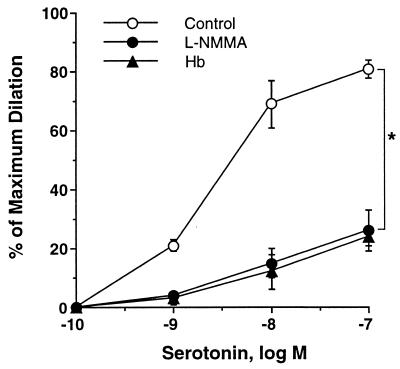
To establish the effect of free Hb on NO-mediated dilation in the absence of flow, the vessel was dilated by serotonin, which has been shown to induce NO-mediated dilation in coronary microvessels (28). After pressurizing the vessel to 60 cm H2O luminal pressure without flow by setting both reservoirs to the same hydrostatic level, serotonin (10−10 M to 10−6 M) was administered cumulatively into the vessel bath, and the concentration-dependent dilation was examined in the absence (vehicle control) and presence of luminal Hb. Results show that free Hb (10 μM) significantly reduced serotonin-induced dilation at doses ranging from 10−10 to 10−7 M (Fig. 1). The effect of free Hb is identical to that of the NO synthase inhibitor, l-NMMA (10 μM), suggesting that the inhibitory effect of free Hb can be attributed to the reduction of NO concentration. These results show that free Hb, indeed, is a potent NO scavenger and functionally inhibits NO-mediated dilation of pressurized microvessels, even at a concentration as low as 10 μM.
Figure 1.
NO mediated vasodilation to serotonin was attenuated by 10 μM oxyHb, giving a response identical to that of NOS inhibitor l-NMMA (10 μM). Basal diameter = 76 ± 2 μm, maximum diameter = 98 ± 3 μm; P < 0.05 versus control.
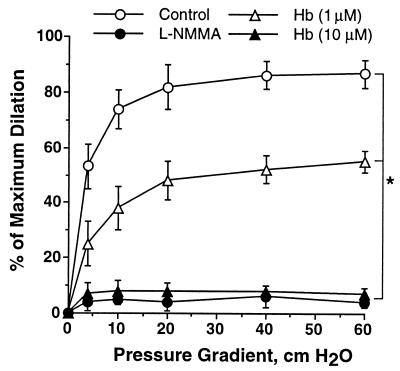
To investigate the effect of flow on NO interaction with free Hb, the vascular response to flow (ΔP = 4, 10, 20, 40, and 60 cm H2O) in the absence and presence of luminal Hb was examined. In the absence of luminal Hb, increasing intravascular flow resulted in dilation of coronary arterioles (Fig. 2). However, this flow-induced dilation was significantly inhibited by 1 μM Hb (expressed in heme concentration) and was abolished by 10 μM Hb. Comparing the effects of free Hb (10 μM) on serotonin-induced and flow-induced dilation, we found that the presence of flow does not affect NO consumption by free Hb. In both cases, 10 μM of free Hb eliminated the NO-mediated dilation, identical to the effect of l-NMMA (Fig. 2).
Figure 2.
Flow-induced vasodilation was attenuated by oxyHb in a dose-dependent manner. Vasodilatory response to the increased flow was completely abolished by 10 μM oxyHb, identical to that of NOS inhibitor l-NMMA (10 μM). n = 5, basal diameter = 74 ± 4 μm; maximum diameter = 112 ± 3 μm; ∗, P < 0.05, all interventions versus control.
Intravascular Flow Reduces the NO Consumption by RBCs.
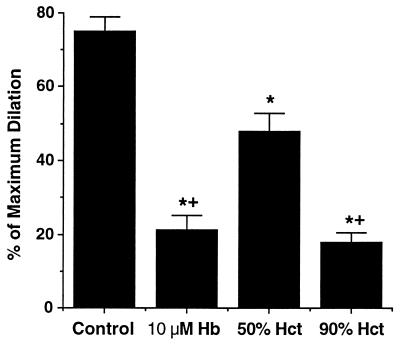
To investigate the effect of flow on NO consumption by RBCs, we examined the influence of RBCs on NO-mediated dilation in the absence and presence of intravascular flow. In the absence of flow, serotonin was used to induce NO production in a vessel containing an RBC suspension in the lumen. Interestingly, the luminal solution containing 50% hematocrit (about 12 mM of overall heme) reduced serotonin-induced dilation by 30% (Fig. 3), much less than the effect of 10 μM of free Hb. To inhibit serotonin-induced dilation to the same extent as free Hb (10 μM), a 90% hematocrit, i.e., about 21 mM of heme, was required (Fig. 3). These results suggest that NO consumption by RBCs is slower than that of free Hb by at least 3 orders of magnitude.
Figure 3.
The vasodilation in response to serotonin (0.1 μM) was significantly inhibited by free oxyHb and RBCs with 50% or 90% hematocrit (corresponding to 3 and 5.6 mM overall Hb, respectively). Fifty percent hematocrit (3.1 mM Hb) had less inhibition to serotonin-induced dilation than 10 μM free oxyHb. n = 5, basal diameter = 76 ± 2 μm, maximum diameter = 98 ± 3 μm; ∗, P < 0.05, versus control; +, P < 0.05, versus 50% hematocrit.
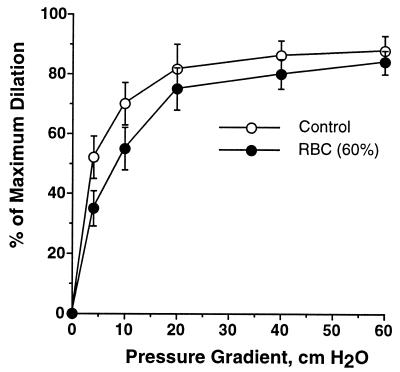
To investigate the effect of RBCs in the presence of intravascular flow, vessel dilation was induced by flow with and without RBC suspension in the perfusion solution. If flow had no effect on the RBC-NO interaction, one would expect that an RBC suspension at about 50% hematocrit should reduce dilation by about 30%, similar to the RBC effect on serotonin-induced dilation. Unexpectedly, when the vessel was perfused with RBCs at 60% hematocrit (corresponding to 14 mM of overall heme concentration), flow-induced vasodilation was not affected (Fig. 4). This result suggests that intravascular flow decreased NO consumption by RBCs. Fig. 5 summarizes the effects of intravascular flow on inhibition of NO-mediated dilation by free Hb and RBCs. It shows that free Hb (10 μM) effectively inhibited NO-mediated dilation in response to either serotonin (10−7 M) or shear stress (ΔP = 60 cm H2O). The presence of flow does not attenuate the effect of free Hb. In contrast, NO-mediated vessel dilation was hardly affected by flowing RBCs (50–60% hematocrit).
Figure 4.
The vasodilatory response to the increased flow (i.e., pressure gradient) during perfusion with a PSS or RBCs. The flow-induced vasodilation, especially at the lower flow rates, was slightly inhibited by RBCs. n = 5, basal diameter = 78 ± 4 μm, maximum diameter = 104 ± 3 μm.
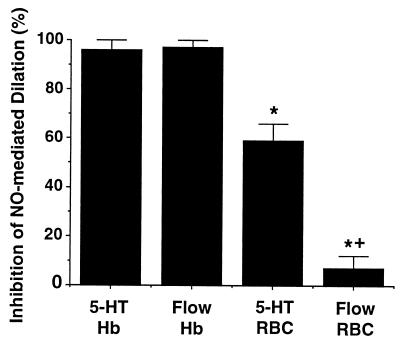
Figure 5.
Summary of the effect of flow on inhibition of vasodilation by free Hb and RBCs from Figs. 1–4. The presence of flow reduced the inhibitory effect of RBCs (50–60% hematocrit) on NO-mediated vessel dilation [+, P < 0.05, serotonin (5-HT) versus flow, ΔP = 60 cm H2O]. In contrast, flow did not change the effect of free Hb. Free Hb (10 μM) effectively inhibited NO-mediated dilation in response to either serotonin (0.1 μM) or shear stress (ΔP = 60 cm H2O), but RBCs (50–60% hematocrit) had significantly less effect. ∗, P < 0.05, RBCs versus free Hb.
DISCUSSION
The above results demonstrate two important findings. First, NO consumption by RBCs is slower than free Hb by about 3 orders of magnitude. Second, intravascular flow further reduces the NO consumption by RBCs. In contrast, intravascular flow does not affect NO consumption by free Hb. The low NO consumption rate by RBCs is consistent with our prediction based on mathematical modeling (14). However, the effect of flow on NO consumption by RBCs is not readily predicted. As described above, intravascular flow may generate two possible effects on NO consumption. The first is the reduction of diffusion distance through more efficient micro mixing. This effect tends to increase the NO consumption by RBCs, inhibiting the vessel dilation. The second is the creation of an RBC-free zone, which reduces the NO consumption by RBCs. The latter effect appears dominant, and thus intravascular flow reduces the scavenging effect of RBC and allows the released NO to exert its vasodilatory function. This RBC-free zone varies with shear stress and can be as much as 20–25% of the lumen diameter in thickness (29). In contrast, free Hb exists as a homogeneous solution with or without flow. Therefore, flow has no effect on NO consumption by free Hb. In addition to the induction and activation of NO synthase by shear stress, intravascular flow reduces NO consumption by RBC and thus generally enhances NO availability for its biological action.
The above results suggest that the RBC-free zone near the vessel wall is important in reducing the NO consumption by RBCs. However, the RBC-free zone does not completely explain the low NO consumption rate by RBCs. Even without this phenomenon (e.g., in serotonin-induced dilation), the NO consumption by RBC is still much lower than free Hb (Fig. 3). This difference also has been observed previously under conditions without the RBC-free zone (20, 30–32).
Fig. 4 demonstrates that under physiological conditions (40–50% hematocrit and in the presence of flow), RBC suspension is not an NO scavenger. Previously, several theories have been proposed to explain why NO is not scavenged by RBCs. Notably, S-nitrosylation of Hb to form Cysβ93-nitrosyl Hb (SNO-Hb) has been proposed to be a mechanism bypassing the NO scavenging problem (19). In this mechanism, Hb was suggested to be an NO carrier rather than an NO scavenger. Gow and Stamler (19) proposed a dynamic sequence as part of blood pressure control. According to this theory, in the veins (deoxy conditions), Hb (T state) binds to NO at the heme site. Upon oxygenation (T to R transition), βFe(II)NO can transfer NO to the β93Cys group to form SNO-Hb (R state). When SNO-Hb circulates to the arterioles and capillaries (deoxy), the SNO group is released to dilate the vessels, and, at the same time, NO produced from local endothelium is bound to the heme group to complete the cycle. Although the SNO-Hb theory is innovative and interesting, it does not address the issue of NO scavenging by Hb. It is recognized and experimentally shown (unpublished results) that S-nitrosylation does not reduce the rate of NO reaction with the oxygen bound to the heme group. Moreover, the rate of the S-nitrosylation reaction is much slower than that of the NO-oxyHb reaction, and thus the majority of NO will be oxidized by oxyHb. Indeed, the SNO-Hb measured in rats (18) was at most in the 400 nM range, 4 orders of magnitude lower than the total Hb in blood (about 12 mM of heme). Therefore, regardless of the physiological role of SNO-Hb, this proposed mechanism alone cannot explain why NO is not degraded by Hb in blood. The success of the SNO-Hb theory thus requires a mechanism to prevent NO from being completely scavenged. The results reported here, along with the undisturbed layer theory (20), may partially provide such missing mechanisms.
Why the RBC does not scavenge NO, even without the RBC-free zone, is still incompletely understood. Liu et al. (20) suggested that the undisturbed layer surrounding RBCs created sufficient diffusion resistance. Yonetani et al. (33) suggest that low concentrations of NO in blood (10−7 to 10−5 M) under partial oxygenation is scavenged by deoxyHb, rather than by oxyHb, to form α(Fe-NO)2β(Fe)2, (kon = 107 M−1⋅s−1). This product is a cooperative, low affinity oxygen carrier that can deliver oxygen to tissue. The α(Fe-NO)2β(Fe)2 eventually is oxidized to nitrate and met-hemes by oxygen with a half-life of about 30 min. This observation may nicely explain the fate of NO under low oxygenation conditions, but does not address the NO scavenging issue. The binding of NO to α-hemes occurs almost as fast as the reaction of NO with oxyHb, and NO eventually is oxidized. Therefore, this theory requires some other mechanisms to reduce the NO uptake rate by RBCs. The extracellular diffusion layer proposed by Liu et al. (20) provides a possible explanation. Here we show that the RBC-free zone is partially responsible for the reduction of NO consumption rate by RBCs. This finding is important because the RBC-free zone is increasingly significant in the microcirculation where blood flow is actually controlled. The reduced NO consumption by RBCs in the microcirculation might enhance the effective NO diffusion distance (14) for vasoregulation.
Acknowledgments
This work was sponsored by American Heart Association Grant 9750273N to J.C.L.
ABBREVIATIONS
- Hb
hemoglobin
- RBC
red blood cell
- l-NMMA
NG-monomethyl-l-arginine
- PSS
physiological salt solution
- SNO-Hb
Cysβ93-nitrosyl Hb
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Fukumura D, Yuan F, Endo M, Jain R K. Am J Pathol. 1997;150:713–725. [PMC free article] [PubMed] [Google Scholar]
- 2.Kelm M, Feelisch M, Krebber T, Deussen A, Motz W, Strauer B E. Hypertension. 1995;25:186–193. doi: 10.1161/01.hyp.25.2.186. [DOI] [PubMed] [Google Scholar]
- 3.Radomski M W, Moncada S. Adv Exp Med Biol. 1993;344:251–264. doi: 10.1007/978-1-4615-2994-1_20. [DOI] [PubMed] [Google Scholar]
- 4.Radomski M W, Palmer R M, Moncada S. Trends Pharmacol Sci. 1991;12:87–88. doi: 10.1016/0165-6147(91)90510-y. [DOI] [PubMed] [Google Scholar]
- 5.Marks D S, Vita J A, Folts J D, Keaney J F J, Welch G N, Loscalzo J. J Clin Invest. 1995;96:2630–2638. doi: 10.1172/JCI118328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soyombo A A, Thurston V J, Newby A C. Eur Heart J. 1993;14:201–206. [PubMed] [Google Scholar]
- 7.Kupatt C, Zahler S, Seligmann C, Massoudy P, Becker B F, Gerlach E. J Mol Cell Cardiol. 1996;28:643–654. doi: 10.1006/jmcc.1996.0060. [DOI] [PubMed] [Google Scholar]
- 8.Rees D D, Palmer R M, Moncada S. Proc Natl Acad Sci USA. 1989;86:3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu A, Chambers D E, Lin C C, Kuehl W D, Palmer R M, Cobb F R. J Clin Invest. 1991;87:1964–1968. doi: 10.1172/JCI115223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenblum W I. Stroke. 1997;28:186–189. doi: 10.1161/01.str.28.1.186. [DOI] [PubMed] [Google Scholar]
- 11.Cassoly R, Gibson Q. J Mol Biol. 1975;91:301–313. doi: 10.1016/0022-2836(75)90382-4. [DOI] [PubMed] [Google Scholar]
- 12.Eich R F, Li T, Lemon D D, Doherty D H, Curry S R, Aitken J F, Mathews A J, Johnson K A, Smith R D, Phillips G N, Jr, Olson J S. Biochemistry. 1996;35:6976–6983. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 13.Lancaster J R. Proc Natl Acad Sci USA. 1994;91:8137–8141. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaughn M W, Kuo L, Liao J C. Am J Physiol. 1998;274:H1705–H1714. doi: 10.1152/ajpheart.1998.274.5.H1705. [DOI] [PubMed] [Google Scholar]
- 15.Hess J R, MacDonald V W, Brinkley W W. J Appl Physiol. 1993;74:1769–1778. doi: 10.1152/jappl.1993.74.4.1769. [DOI] [PubMed] [Google Scholar]
- 16.Evans H G, Ryley H C, Hallett I, Lewis M J. Eur J Pharmacol. 1989;163:361–364. doi: 10.1016/0014-2999(89)90207-0. [DOI] [PubMed] [Google Scholar]
- 17.Mosseri M, Bartlett-Pandite A N, Wenc K, Isner J W. Am Heart J. 1993;126:338–346. doi: 10.1016/0002-8703(93)91049-k. [DOI] [PubMed] [Google Scholar]
- 18.Jia L, Bonaventura C, Bonaventura J, Stamler J S. Nature (London) 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 19.Gow A J, Stamler J S. Nature (London) 1998;391:169–173. doi: 10.1038/34402. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Miller M S, Joshi M S, Sadowska-Krowicka H, Clark D A, Lancaster J R. J Biol Chem. 1998;273:18709–18713. doi: 10.1074/jbc.273.30.18709. [DOI] [PubMed] [Google Scholar]
- 21.Butler A R, Megson I L, Wright P G. Biochim Biophys Acta. 1998;1425:168–176. doi: 10.1016/s0304-4165(98)00065-8. [DOI] [PubMed] [Google Scholar]
- 22.Kuo L, Davis M J, Chilian W M. Am J Physiol. 1988;255:H1558–H1562. doi: 10.1152/ajpheart.1988.255.6.H1558. [DOI] [PubMed] [Google Scholar]
- 23.Kuo L, Chilian W M, Davis M J. Am J Physiol. 1991;2661:H1706–H1715. doi: 10.1152/ajpheart.1991.261.6.H1706. [DOI] [PubMed] [Google Scholar]
- 24.Chilian W M, Layne S M, Nellis S H. In: Coronary Circulation: Basic Mechanisms and Clinical Relevance. Kajiya F, Klassen G A, Spaan J A E, Hoffman J I E, editors. New York: Springer; 1990. pp. 173–187. [Google Scholar]
- 25.Kuo L, Davis M J, Chilian W M. Am J Physiol. 1990;259:H1063–H1070. doi: 10.1152/ajpheart.1990.259.4.H1063. [DOI] [PubMed] [Google Scholar]
- 26.Beutler E. Red Cell Metabolism: A Manual of Biochemical Methods. Orlando: Grune and Stratton; 1984. [Google Scholar]
- 27.Di Iorio E E. Methods Enzymol. 1981;76:57–71. doi: 10.1016/0076-6879(81)76114-7. [DOI] [PubMed] [Google Scholar]
- 28.Hein T W, Kuo L. Circ Res. 1998;83:404–414. doi: 10.1161/01.res.83.4.404. [DOI] [PubMed] [Google Scholar]
- 29.Schmid-Schönbein H, Fisher T, Driessen G, Rieger H. In: Quantitative Cardiovascular Studies: Clinical Research Application of Engineering Principles. Hwang N H C, Gross D R, Patel D J, editors. Baltimore: Univ. Park Press; 1979. pp. 353–417. [Google Scholar]
- 30.Gillespie J S, Sheng H. Br J Pharmacol. 1989;98:445–450. doi: 10.1111/j.1476-5381.1989.tb12616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kilbourn R G, Joly G, Cashon B, DeAngelo J, Bonaventura J. Biochem Biophys Res Commun. 1994;199:155–162. doi: 10.1006/bbrc.1994.1208. [DOI] [PubMed] [Google Scholar]
- 32.Rioux F, Petitclerc E, Audet R, Drapeau G, Fielding R M, Marceau F. J Cardiovasc Pharmacol. 1994;24:229–237. [PubMed] [Google Scholar]
- 33.Yonetani T, Tsuneshige A, Zhou Y, Chen X. J Biol Chem. 1998;273:20323–20333. doi: 10.1074/jbc.273.32.20323. [DOI] [PubMed] [Google Scholar]