Abstract
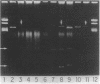
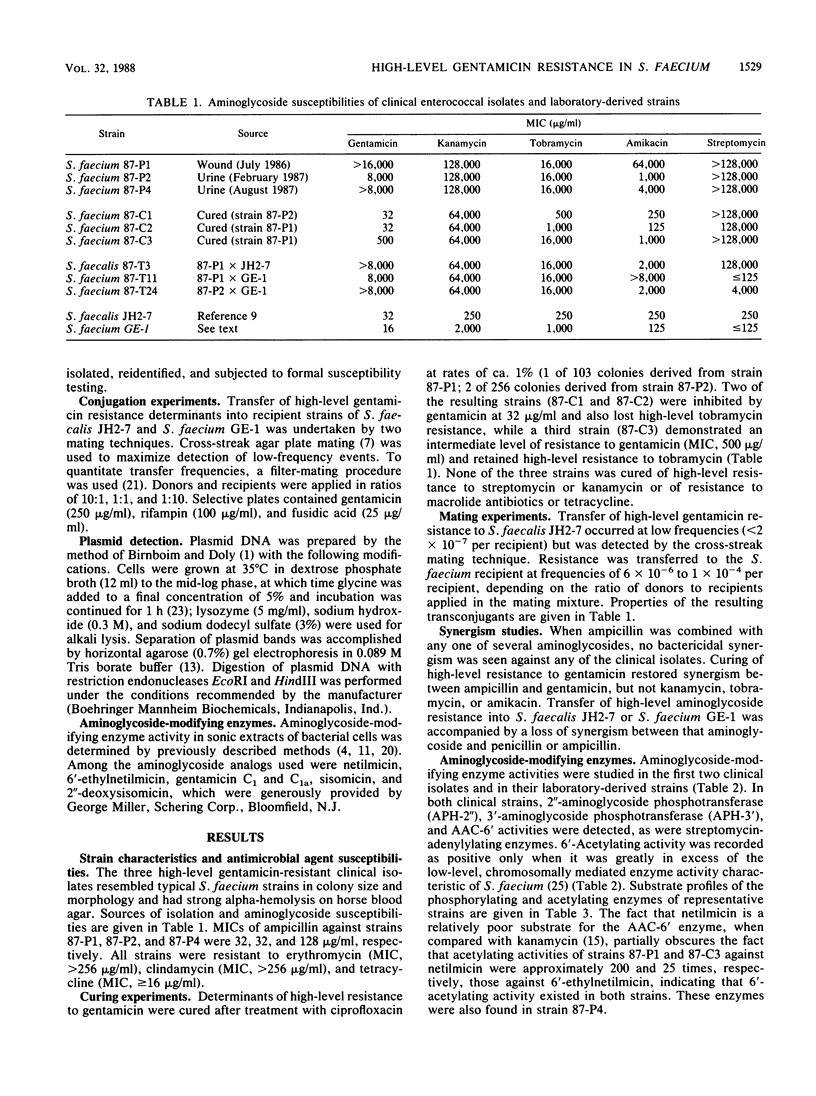
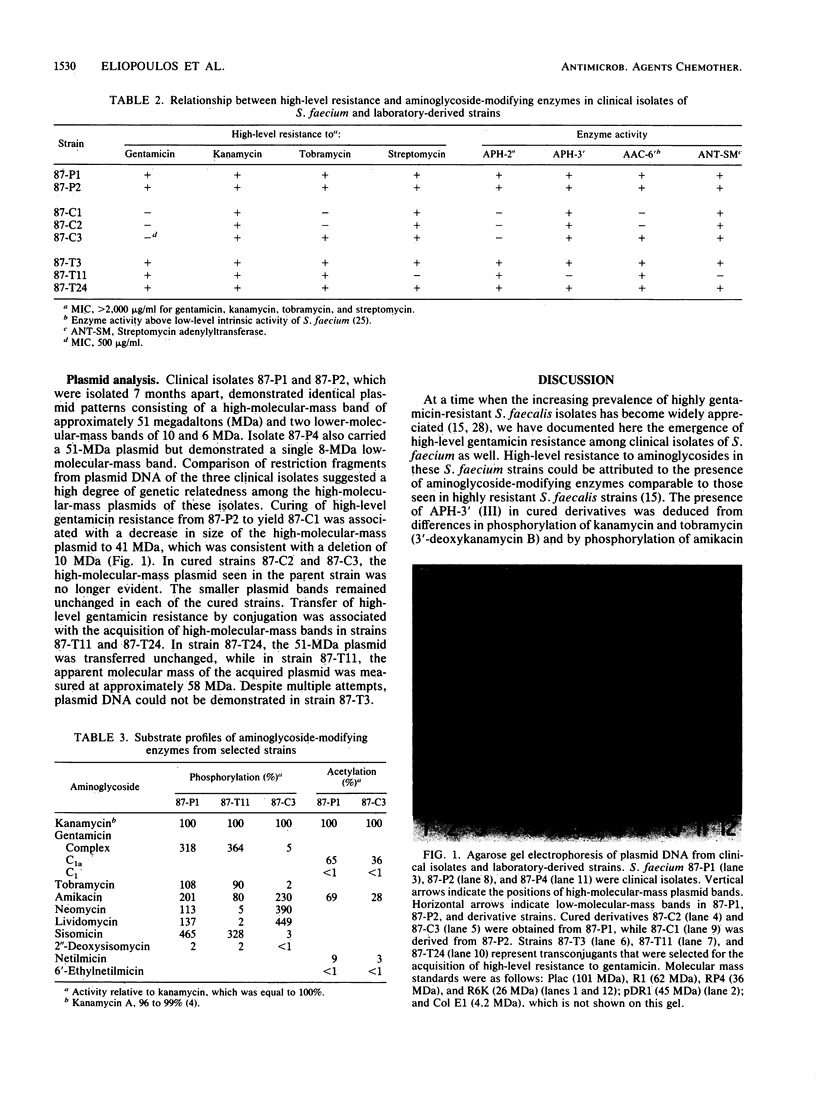
During a 14-month period beginning in July 1986, three distinct clinical isolates of Streptococcus (Enterococcus) faecium demonstrating high-level resistance (MIC, greater than 2,000 micrograms/ml) to gentamicin, kanamycin, tobramycin, and streptomycin were recovered from individual patients at one institution. Combinations of ampicillin with any of these agents failed to show bactericidal synergism. By filter-mating techniques, high-level gentamicin resistance could be transferred into a susceptible recipient of the same species at frequencies as high as 1 x 10(-4); transfer into Streptococcus faecalis JH2-7 occurred at lower frequencies (less than 2 x 10(-7). Aminoglycoside substrate profile analysis of clinical isolates as well as of laboratory-derived cured strains and transconjugants revealed 2"-aminoglycoside phosphotransferase and 3'-aminoglycoside phosphotransferase (III) phosphorylating enzymes, AAC-6' acetylating activity above that attributable to the intrinsic activity characteristic of S. faecium, and a streptomycin adenylylating enzyme. All three isolates carried a 51-megadalton plasmid. Curing of this plasmid or conjugative transfer into susceptible recipients was associated with the loss or acquisition of high-level gentamicin resistance, respectively. Loss of high-level gentamicin resistance was also observed when curing techniques resulted in a decrease in the size of this plasmid equivalent to a 10-megadalton deletion. Transferable, high-level resistance to gentamicin and other aminoglycosides, which was previously recognized in S. faecalis, has now emerged in clinical isolates of S. faecium, with the attendant concerns for possible spread.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. Y., Williams J. D. Transferable resistance and aminoglycoside-modifying enzymes in enterococci. J Med Microbiol. 1985 Oct;20(2):187–196. doi: 10.1099/00222615-20-2-187. [DOI] [PubMed] [Google Scholar]
- Combes T., Carlier C., Courvalin P. Aminoglycoside-modifying enzyme content of a multiply resistant strain of Streptococcus faecalis. J Antimicrob Chemother. 1983 Jan;11(1):41–47. doi: 10.1093/jac/11.1.41. [DOI] [PubMed] [Google Scholar]
- Courvalin P., Carlier C. Resistance towards aminoglycoside-aminocyclitol antibiotics in bacteria. J Antimicrob Chemother. 1981 Jul;8 (Suppl A):57–69. doi: 10.1093/jac/8.suppl_a.57. [DOI] [PubMed] [Google Scholar]
- Eliopoulos G. M., Wennersten C., Moellering R. C., Jr Resistance to beta-lactam antibiotics in Streptococcus faecium. Antimicrob Agents Chemother. 1982 Aug;22(2):295–301. doi: 10.1128/aac.22.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti J. J., Gilmore K. S., Courvalin P. Nucleotide sequence analysis of the gene specifying the bifunctional 6'-aminoglycoside acetyltransferase 2"-aminoglycoside phosphotransferase enzyme in Streptococcus faecalis and identification and cloning of gene regions specifying the two activities. J Bacteriol. 1986 Aug;167(2):631–638. doi: 10.1128/jb.167.2.631-638.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A. E., Clewell D. B. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of "conjugal" transfer in the absence of a conjugative plasmid. J Bacteriol. 1981 Jan;145(1):494–502. doi: 10.1128/jb.145.1.494-502.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodniceanu T., Bougueleret L., El-Solh N., Bieth G., Delbos F. High-level, plasmid-borne resistance to gentamicin in Streptococcus faecalis subsp. zymogenes. Antimicrob Agents Chemother. 1979 Nov;16(5):686–689. doi: 10.1128/aac.16.5.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogstad D. J., Korfhagen T. R., Moellering R. C., Jr, Wennersten C., Swartz M. N. Aminoglycoside-inactivating enzymes in clinical isolates of Streptococcus faecalis. An explanation for resistance to antibiotic synergism. J Clin Invest. 1978 Aug;62(2):480–486. doi: 10.1172/JCI109149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bouguenec C., Horodniceanu T. Conjugative R plasmids in Streptococcus faecium (group D). Antimicrob Agents Chemother. 1982 May;21(5):698–705. doi: 10.1128/aac.21.5.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh G. L., Swartz M. N. Elimination of plasmids from several bacterial species by novobiocin. Antimicrob Agents Chemother. 1977 Sep;12(3):423–426. doi: 10.1128/aac.12.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mederski-Samoraj B. D., Murray B. E. High-level resistance to gentamicin in clinical isolates of enterococci. J Infect Dis. 1983 Apr;147(4):751–757. doi: 10.1093/infdis/147.4.751. [DOI] [PubMed] [Google Scholar]
- Michel-Briand Y., Uccelli V., Laporte J. M., Plesiat P. Elimination of plasmids from Enterobacteriaceae by 4-quinolone derivatives. J Antimicrob Chemother. 1986 Dec;18(6):667–674. doi: 10.1093/jac/18.6.667. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Korzeniowski O. M., Sande M. A., Wennersten C. B. Species-specific resistance to antimocrobial synergism in Streptococcus faecium and Streptococcus faecalis. J Infect Dis. 1979 Aug;140(2):203–208. doi: 10.1093/infdis/140.2.203. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Mederski-Samaroj B. Transferable beta-lactamase. A new mechanism for in vitro penicillin resistance in Streptococcus faecalis. J Clin Invest. 1983 Sep;72(3):1168–1171. doi: 10.1172/JCI111042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Moellering R. C., Jr Aminoglycoside-modifying enzymes among clinical isolates of Acinetobacter calcoaceticus subsp. anitratus (Herellea vaginicola): explanation for high-level aminoglycoside resistance. Antimicrob Agents Chemother. 1979 Feb;15(2):190–199. doi: 10.1128/aac.15.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Moellering R. C., Jr Evidence of plasmid-mediated production of aminoglycoside-modifying enzymes not previously described in Acinetobacter. Antimicrob Agents Chemother. 1980 Jan;17(1):30–36. doi: 10.1128/aac.17.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Tsao J., Panida J. Enterococci from Bangkok, Thailand, with high-level resistance to currently available aminoglycosides. Antimicrob Agents Chemother. 1983 Jun;23(6):799–802. doi: 10.1128/aac.23.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper K., Horaud T., Le Bouguénec C., de Cespédès G. Location of antibiotic resistance markers in clinical isolates of Enterococcus faecalis with similar antibiotypes. Antimicrob Agents Chemother. 1987 Sep;31(9):1394–1402. doi: 10.1128/aac.31.9.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieu-Cuot P., Courvalin P. Evolution and transfer of aminoglycoside resistance genes under natural conditions. J Antimicrob Chemother. 1986 Oct;18 (Suppl 100):93–102. doi: 10.1093/jac/18.supplement_c.93. [DOI] [PubMed] [Google Scholar]
- Williamson R., Calderwood S. B., Moellering R. C., Jr, Tomasz A. Studies on the mechanism of intrinsic resistance to beta-lactam antibiotics in group D streptococci. J Gen Microbiol. 1983 Mar;129(3):813–822. doi: 10.1099/00221287-129-3-813. [DOI] [PubMed] [Google Scholar]
- Zervos M. J., Dembinski S., Mikesell T., Schaberg D. R. High-level resistance to gentamicin in Streptococcus faecalis: risk factors and evidence for exogenous acquisition of infection. J Infect Dis. 1986 Jun;153(6):1075–1083. doi: 10.1093/infdis/153.6.1075. [DOI] [PubMed] [Google Scholar]
- Zervos M. J., Kauffman C. A., Therasse P. M., Bergman A. G., Mikesell T. S., Schaberg D. R. Nosocomial infection by gentamicin-resistant Streptococcus faecalis. An epidemiologic study. Ann Intern Med. 1987 May;106(5):687–691. doi: 10.7326/0003-4819-106-5-687. [DOI] [PubMed] [Google Scholar]