Abstract
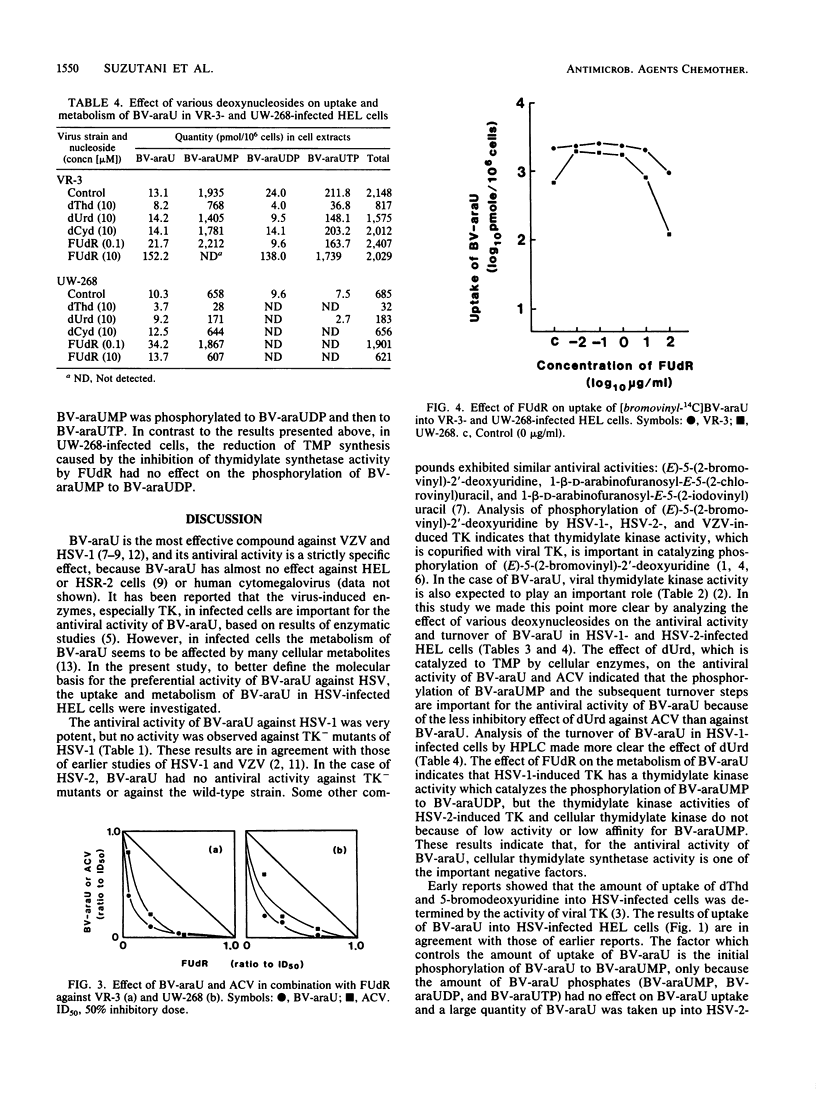
Uptake of 1-beta-D-arabinofuranosyl-E-5-(2-bromovinyl)uracil (BV-araU) into herpes simplex virus type 1 (HSV-1)- and 2 (HSV-2)-infected cells was elevated about 190 to 40 times, compared with that into mock-infected human embryo lung fibroblast cells. Uptake was not enhanced by infection with thymidine kinase-negative HSV-1 and HSV-2 mutants, however. In HSV-1-infected cells, 9.7% of BV-araU was phosphorylated to BV-araU triphosphate, but only 1.1% was phosphorylated in HSV-2-infected cells. The antiviral effect, uptake, and turnover of BV-araU were inhibited significantly by thymidine (dThd), moderately by deoxyuridine, and not at all by deoxycytidine. On the other hand, the antiviral activity of acyclovir (ACV) was inhibited only by dThd. The effect of BV-araU was influenced by dThd and dThd phosphates (mono-, di-, and triphosphates), and the effect of ACV was influenced only by dThd, which competitively inhibited the phosphorylation of ACV to ACV monophosphate. The combination of 5-fluorodeoxyuridine (FUdR)-BV-araU or FUdR-ACV had a synergistic effect on HSV-1 and HSV-2 replication. The effect of FUdR on the turnover of BV-araU in HSV-1- and HSV-2-infected cells was analyzed by high-performance liquid chromatography. In HSV-1-infected cells, 86% of the BV-araU was phosphorylated to BV-araU triphosphate, but no effect was observed in HSV-2-infected cells, in which 98% of the BV-araU remained as BV-araU monophosphate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayisi N. K., De Clercq E., Wall R. A., Hughes H., Sacks S. L. Metabolic fate of (E)-5-(2-bromovinyl)-2'-deoxyuridine in herpes simplex virus- and mock-infected cells. Antimicrob Agents Chemother. 1984 Nov;26(5):762–765. doi: 10.1128/aac.26.5.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayisi N. K., Wall R. A., Wanklin R. J., Machida H., De Clercq E., Sacks S. L. Comparative metabolism of E-5-(2-bromovinyl)-2'-deoxyuridine and 1-beta-D-arabinofuranosyl-E-5-(2-bromovinyl)uracil in herpes simplex virus-infected cells. Mol Pharmacol. 1987 Apr;31(4):422–429. [PubMed] [Google Scholar]
- Bittlingmaier K., Schneider D., Falke D. Thymidine transport in herpesvirus hominis type 1 and 2 infected BHK 21 cells. J Gen Virol. 1977 Apr;35(1):159–173. doi: 10.1099/0022-1317-35-1-159. [DOI] [PubMed] [Google Scholar]
- Chen M. S., Amico L. A., Speelman D. J. Kinetics of the interaction of monophosphates of the antiviral nucleosides 2'-fluoro-1-beta-D-arabinofuranosylpyrimidine and (E)-5-(2-bromovinyl)-2'-deoxyuridine with thymidylate kinases from Vero cells and herpes simplex virus types 1 and 2. Antimicrob Agents Chemother. 1984 Nov;26(5):778–780. doi: 10.1128/aac.26.5.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Dutschman G., Fox J. J., Watanabe K. A., Machida H. Differential activity of potential antiviral nucleoside analogs on herpes simplex virus-induced and human cellular thymidine kinases. Antimicrob Agents Chemother. 1981 Sep;20(3):420–423. doi: 10.1128/aac.20.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe J. A. Differential phosphorylation of (E)-5-(2-bromovinyl)-2'-deoxyuridine monophosphate by thymidylate kinases from herpes simplex viruses types 1 and 2 and varicella zoster virus. Mol Pharmacol. 1982 Mar;21(2):432–437. [PubMed] [Google Scholar]
- Machida H. Comparison of susceptibilities of varicella-zoster virus and herpes simplex viruses to nucleoside analogs. Antimicrob Agents Chemother. 1986 Mar;29(3):524–526. doi: 10.1128/aac.29.3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida H., Kuninaka A., Yoshino H. Inhibitory effects of antiherpesviral thymidine analogs against varicella-zoster virus. Antimicrob Agents Chemother. 1982 Feb;21(2):358–361. doi: 10.1128/aac.21.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida H., Sakata S., Kuninaka A., Yoshino H. Antiherpesviral and anticellular effects of 1-beta-D-arabinofuranosyl-E-5-(2-halogenovinyl) uracils. Antimicrob Agents Chemother. 1981 Jul;20(1):47–52. doi: 10.1128/aac.20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma T. Strains of varicella-zoster virus resistant to 1-beta-D-arabinofuranosyl-E-5-(2-bromovinyl)uracil. Antimicrob Agents Chemother. 1984 Jun;25(6):742–746. doi: 10.1128/aac.25.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeta S., Yokota T., Iwabuchi T., Baba M., Konno K., Ogata M., De Clercq E. Comparative efficacy of antiherpes drugs against various strains of varicella-zoster virus. J Infect Dis. 1983 Mar;147(3):576–584. doi: 10.1093/infdis/147.3.576. [DOI] [PubMed] [Google Scholar]
- Suzutani T., Machida H., Sakuma T. Efficacies of antiherpesvirus nucleosides against two strains of herpes simplex virus type 1 in Vero and human embryo lung fibroblast cells. Antimicrob Agents Chemother. 1988 Jul;32(7):1046–1052. doi: 10.1128/aac.32.7.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]



