Abstract
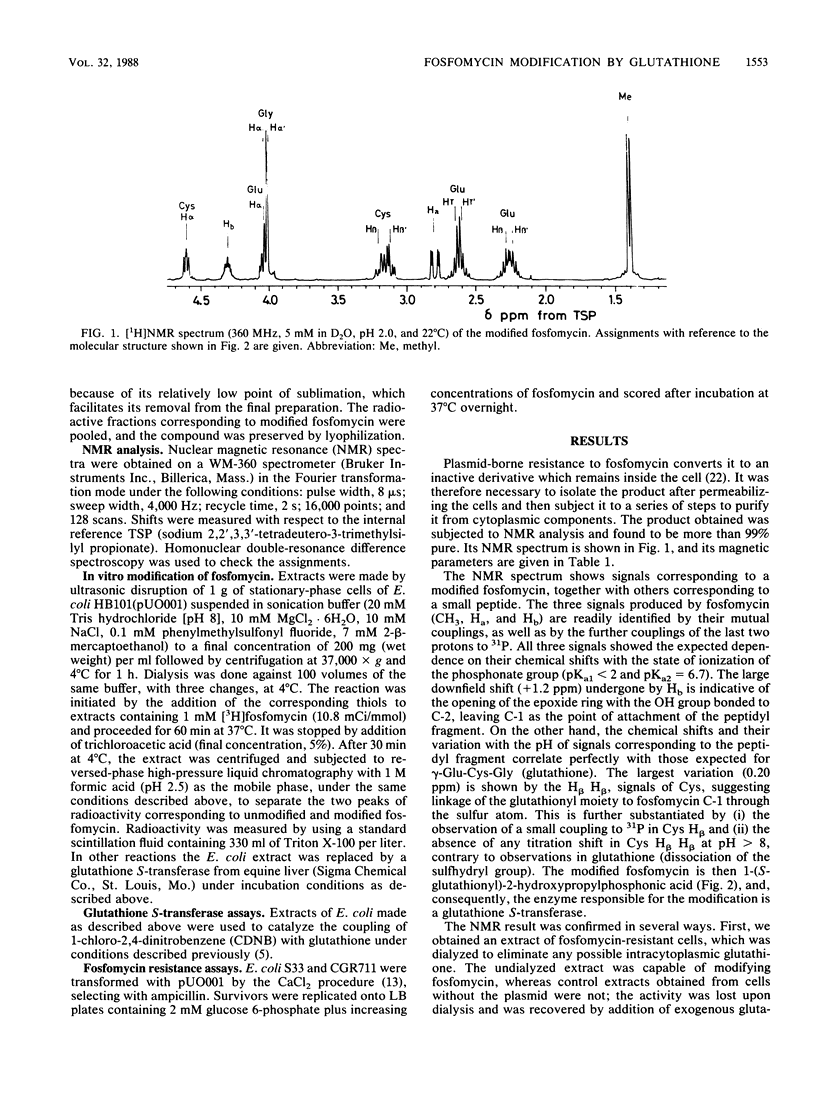
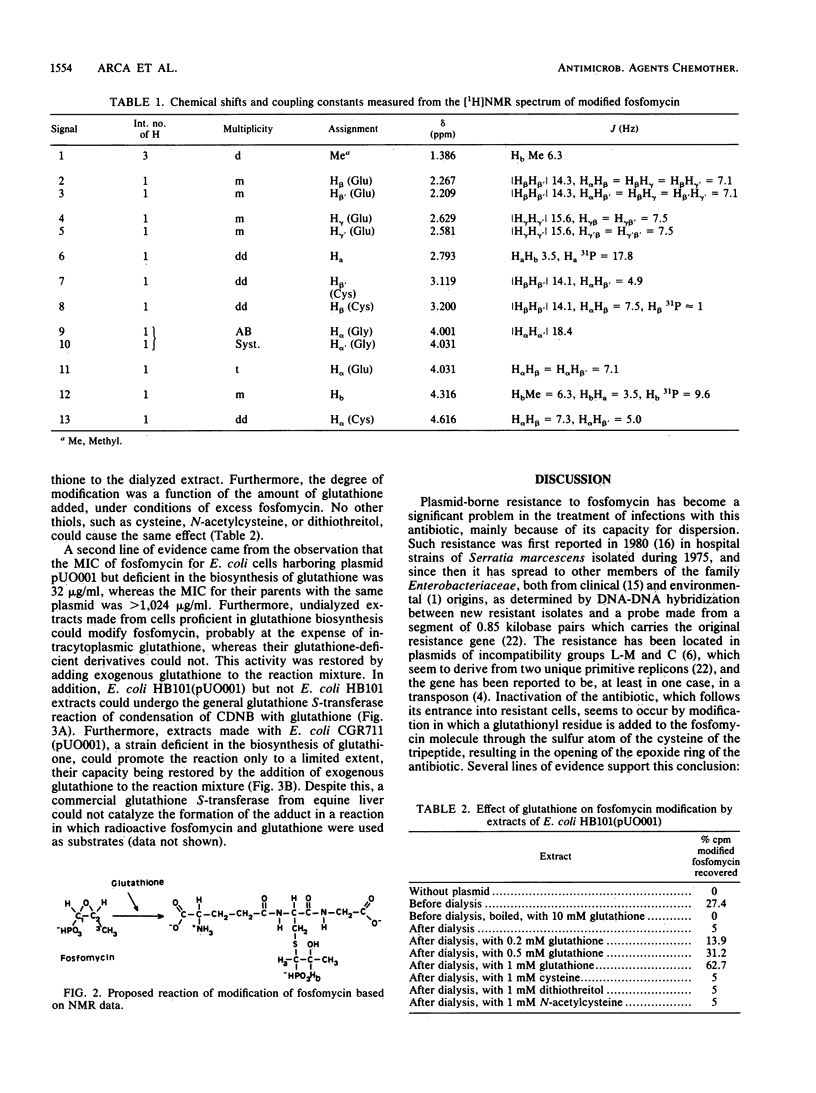
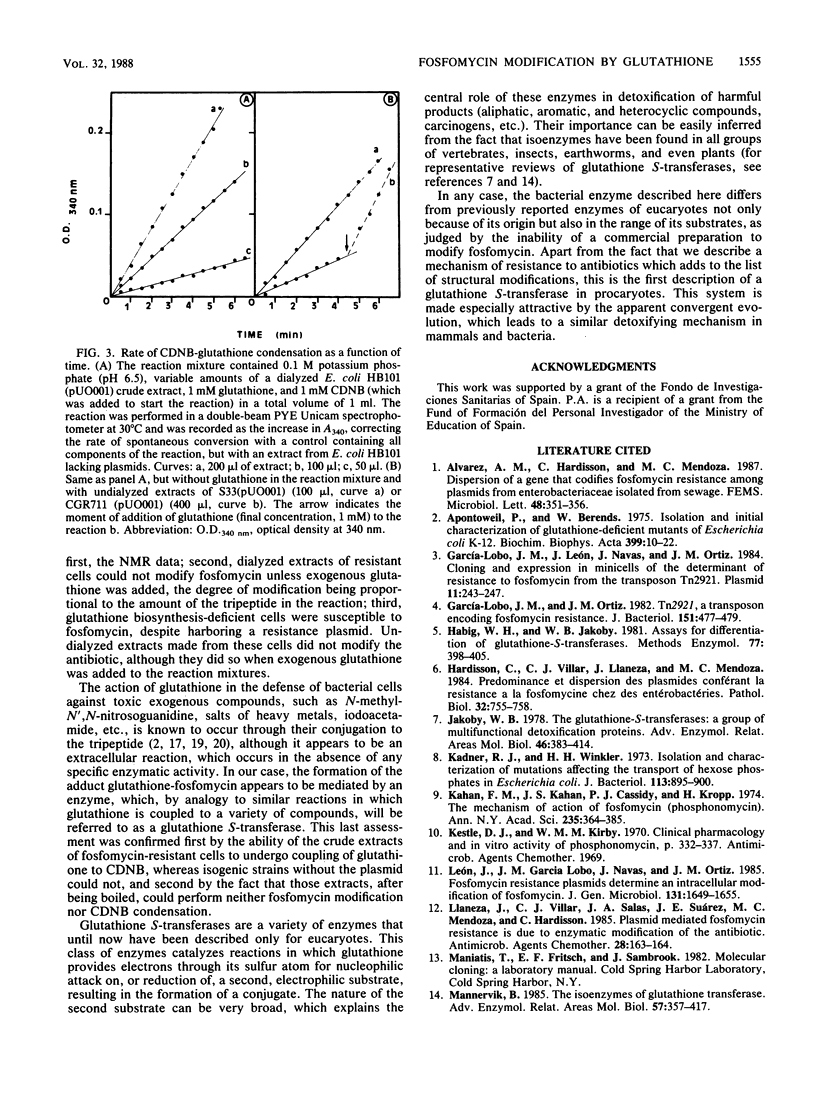
Plasmid-borne resistance to fosfomycin in bacteria is due to modification of the antibiotic molecule by a glutathione S-transferase that catalyzes the formation of a covalent bond between the sulfhydryl residue of the cysteine in glutathione and the C-1 of fosfomycin. This reaction results in opening of the epoxide ring of the antibiotic to form an inactive adduct, the structure of which was confirmed by nuclear magnetic resonance. Dialyzed extracts prepared from resistant Escherichia coli strains were unable to modify fosfomycin unless exogenous glutathione was added to the reaction mixtures. Similarly, mutants defective in glutathione biosynthesis were susceptible to fosfomycin, despite harboring a resistance plasmid. Extracts of resistant but not susceptible strains could join glutathione to 1-chloro-2,4-dinitrobenzene, confirming the nature of the enzymatic activity. Adduct formation appeared to be specific for glutathione: none of the other thiols tested (cysteine, N-acetylcysteine, and dithiothreitol) could modify fosfomycin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apontoweil P., Berends W. Isolation and initial characterization of glutathione-deficient mutants of Escherichia coli K 12. Biochim Biophys Acta. 1975 Jul 14;399(1):10–22. doi: 10.1016/0304-4165(75)90206-8. [DOI] [PubMed] [Google Scholar]
- García-Lobo J. M., León J., Navas J., Ortiz J. M. Cloning and expression in minicells of the determinant of resistance to fosfomycin from the transposon Tn2921. Plasmid. 1984 May;11(3):243–247. doi: 10.1016/0147-619x(84)90030-1. [DOI] [PubMed] [Google Scholar]
- García-Lobo J. M., Ortiz J. M. Tn292l, a transposon encoding fosfomycin resistance. J Bacteriol. 1982 Jul;151(1):477–479. doi: 10.1128/jb.151.1.477-479.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Hardisson C., Villar C. J., Llaneza J., Mendoza M. C. Prédominance et dispersion des plasmides conférant la résistance á la fosfomycine chez des entérobactéries. Pathol Biol (Paris) 1984 Sep;32(7):755–758. [PubMed] [Google Scholar]
- Jakoby W. B. The glutathione S-transferases: a group of multifunctional detoxification proteins. Adv Enzymol Relat Areas Mol Biol. 1978;46:383–414. doi: 10.1002/9780470122914.ch6. [DOI] [PubMed] [Google Scholar]
- Kadner R. J., Winkler H. H. Isolation and characterization of mutations affecting the transport of hexose phosphates in Escherichia coli. J Bacteriol. 1973 Feb;113(2):895–900. doi: 10.1128/jb.113.2.895-900.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan F. M., Kahan J. S., Cassidy P. J., Kropp H. The mechanism of action of fosfomycin (phosphonomycin). Ann N Y Acad Sci. 1974 May 10;235(0):364–386. doi: 10.1111/j.1749-6632.1974.tb43277.x. [DOI] [PubMed] [Google Scholar]
- León J., García-Lobo J. M., Navas J., Ortiz J. M. Fosfomycin-resistance plasmids determine an intracellular modification of fosfomycin. J Gen Microbiol. 1985 Jul;131(7):1649–1655. doi: 10.1099/00221287-131-7-1649. [DOI] [PubMed] [Google Scholar]
- Llaneza J., Villar C. J., Salas J. A., Suarez J. E., Mendoza M. C., Hardisson C. Plasmid-mediated fosfomycin resistance is due to enzymatic modification of the antibiotic. Antimicrob Agents Chemother. 1985 Jul;28(1):163–164. doi: 10.1128/aac.28.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Mendez F. J., Alvarez A. A., Mendoza M. C., Hardisson C. Antibacterial activity of fosmidomycin on chromosomic and plasmid-determined fosfomycin-resistant strains. Chemioterapia. 1985 Apr;4(2):170–175. [PubMed] [Google Scholar]
- Mendoza C., Garcia J. M., Llaneza J., Mendez F. J., Hardisson C., Ortiz J. M. Plasmid-determined resistance to fosfomycin in Serratia marcescens. Antimicrob Agents Chemother. 1980 Aug;18(2):215–219. doi: 10.1128/aac.18.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R. A., Hartman P. E. Glutathione: a protective agent in Salmonella typhimurium and Escherichia coli as measured by mutagenicity and by growth delay assays. Environ Mutagen. 1986;8(5):659–673. doi: 10.1002/em.2860080503. [DOI] [PubMed] [Google Scholar]
- Rodríguez A., Gallego A., Olay T., Mata J. M. Bacteriological evaluation of fosfomycin in clinical studies. Chemotherapy. 1977;23 (Suppl 1):247–258. doi: 10.1159/000222055. [DOI] [PubMed] [Google Scholar]
- Schmidt M. G., Konetzka W. A. Glutathione overproduction by selenite-resistant Escherichia coli. Can J Microbiol. 1986 Oct;32(10):825–827. doi: 10.1139/m86-152. [DOI] [PubMed] [Google Scholar]
- Summer K. H., Göggelmann W. Mutagenicity of 1-fluoro-2,4-dinitrobenzene is affected by bacterial glutathione. Mutat Res. 1980 Apr;70(2):173–178. doi: 10.1016/0027-5107(80)90157-8. [DOI] [PubMed] [Google Scholar]
- Tsuruoka T., Yamada Y. Charactertization of spontaneous fosfomycin (phosphonomycin)-resistant cells of Escherichia coli B in vitro. J Antibiot (Tokyo) 1975 Nov;28(11):906–911. doi: 10.7164/antibiotics.28.906. [DOI] [PubMed] [Google Scholar]
- Villar C. J., Hardisson C., Suárez J. E. Cloning and molecular epidemiology of plasmid-determined fosfomycin resistance. Antimicrob Agents Chemother. 1986 Feb;29(2):309–314. doi: 10.1128/aac.29.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]


