Abstract
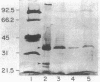
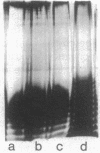
Since beta-lactam resistance is a feature of Pseudomonas cepacia isolates causing pulmonary infections in cystic fibrosis (CF), this study was undertaken to determine whether alterations in beta-lactam permeability mediate drug resistance in this species. A beta-lactam-susceptible non-CF isolate (strain 75-26), a resistant mutant derived from 75-26 by selection for cross-resistance to ciprofloxacin and ceftazidime, and two resistant CF isolates of P. cepacia were used. Permeability constants were calculated from the rate of nitrocefin hydrolysis in intact bacterial cells. Qualitative changes in outer membrane proteins were determined electrophoretically. The permeability constants of the mutant and the resistant CF isolates were lower than the value for the reference strain, 75-26. Whereas the lipopolysaccharide side chains were present in the test and reference strains, the resistant mutant and the CF isolates contained reduced amounts of the 36-kilodalton (kDa) outer membrane protein and failed to express the 27-kDa outer membrane protein. These observations suggest that the 27-kDa outer membrane protein may be a major porin or a major protein component of the porin complex in P. cepacia and that decreased expression of the 36-kDa outer membrane and loss of the 27-kDa porin are associated with high-level beta-lactam resistance in some CF isolates of P. cepacia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus B. L., Carey A. M., Caron D. A., Kropinski A. M., Hancock R. E. Outer membrane permeability in Pseudomonas aeruginosa: comparison of a wild-type with an antibiotic-supersusceptible mutant. Antimicrob Agents Chemother. 1982 Feb;21(2):299–309. doi: 10.1128/aac.21.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff S. C. Derepressed beta-lactamase production as a mediator of high-level beta-lactam resistance in Pseudomonas cepacia. Pediatr Pulmonol. 1988;4(2):72–77. doi: 10.1002/ppul.1950040203. [DOI] [PubMed] [Google Scholar]
- Aronoff S. C., Klinger J. D. Comparison of cefpiramide (HR-810) and four anti-pseudomonal beta-lactam agents against pseudomonas isolates from children with cystic fibrosis. J Antimicrob Chemother. 1985 May;15(5):545–549. doi: 10.1093/jac/15.5.545. [DOI] [PubMed] [Google Scholar]
- Aronoff S. C., Labrozzi P. H. Differences in drug susceptibility between isolates of Pseudomonas cepacia recovered from patients with cystic fibrosis and other sources and its relationship to beta-lactamase focusing pattern. Pediatr Pulmonol. 1986 Nov-Dec;2(6):368–372. doi: 10.1002/ppul.1950020609. [DOI] [PubMed] [Google Scholar]
- Aronoff S. C., Stern R. C. Serum IgG antibody to outer membrane antigens of Pseudomonas cepacia and Pseudomonas aeruginosa in cystic fibrosis. J Infect Dis. 1988 May;157(5):934–940. doi: 10.1093/infdis/157.5.934. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bryan L. E., O'Hara K., Wong S. Lipopolysaccharide changes in impermeability-type aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984 Aug;26(2):250–255. doi: 10.1128/aac.26.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa C., Labrozzi P. H., Aronoff S. C. Decreased baseline beta-lactamase production and inducibility associated with increased piperacillin susceptibility of Pseudomonas cepacia isolated from children with cystic fibrosis. Pediatr Res. 1986 Nov;20(11):1174–1177. doi: 10.1203/00006450-198611000-00026. [DOI] [PubMed] [Google Scholar]
- Conrad D. A., Scribner R. K., Weber A. H., Marks M. I. In vitro activity of BMY-28142 against pediatric pathogens, including isolates from cystic fibrosis sputum. Antimicrob Agents Chemother. 1985 Jul;28(1):58–63. doi: 10.1128/aac.28.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey A. J., Hatlelid L., Bryan L. E. Correlation between lipopolysaccharide structure and permeability resistance in beta-lactam-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984 Aug;26(2):181–186. doi: 10.1128/aac.26.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey A. J., Shahrabadi M. S., Bryan L. E. Distribution of porin and lipopolysaccharide antigens on a Pseudomonas aeruginosa permeability mutant. Antimicrob Agents Chemother. 1986 Nov;30(5):802–805. doi: 10.1128/aac.30.5.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isles A., Maclusky I., Corey M., Gold R., Prober C., Fleming P., Levison H. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr. 1984 Feb;104(2):206–210. doi: 10.1016/s0022-3476(84)80993-2. [DOI] [PubMed] [Google Scholar]
- Klinger J. D., Aronoff S. C. In-vitro activity of ciprofloxacin and other antibacterial agents against Pseudomonas aeruginosa and Pseudomonas cepacia from cystic fibrosis patients. J Antimicrob Chemother. 1985 Jun;15(6):679–684. doi: 10.1093/jac/15.6.679. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Pseudomonas aeruginosa outer membrane permeability: isolation of a porin protein F-deficient mutant. J Bacteriol. 1983 Jan;153(1):281–285. doi: 10.1128/jb.153.1.281-285.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr T. R., Jr, Moore R. A., Moore L. V., Hancock R. E. Role of porins in intrinsic antibiotic resistance of Pseudomonas cepacia. Antimicrob Agents Chemother. 1987 Jan;31(1):121–123. doi: 10.1128/aac.31.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Goering R. V., Werner V. Selection of multiple antibiotic resistance by quinolones, beta-lactams, and aminoglycosides with special reference to cross-resistance between unrelated drug classes. Antimicrob Agents Chemother. 1984 Dec;26(6):797–801. doi: 10.1128/aac.26.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Matsuba K., Yamagishi S. A method for measuring the outer membrane-permeability of beta-lactam antibiotics in gram-negative bacteria. J Antibiot (Tokyo) 1977 Dec;30(12):1134–1136. doi: 10.7164/antibiotics.30.1134. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Examination of the protein composition of the cell envelope of Escherichia coli by polyacrylamide gel electrophoresis. J Bacteriol. 1970 Nov;104(2):882–889. doi: 10.1128/jb.104.2.882-889.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tablan O. C., Chorba T. L., Schidlow D. V., White J. W., Hardy K. A., Gilligan P. H., Morgan W. M., Carson L. A., Martone W. J., Jason J. M. Pseudomonas cepacia colonization in patients with cystic fibrosis: risk factors and clinical outcome. J Pediatr. 1985 Sep;107(3):382–387. doi: 10.1016/s0022-3476(85)80511-4. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]