Abstract
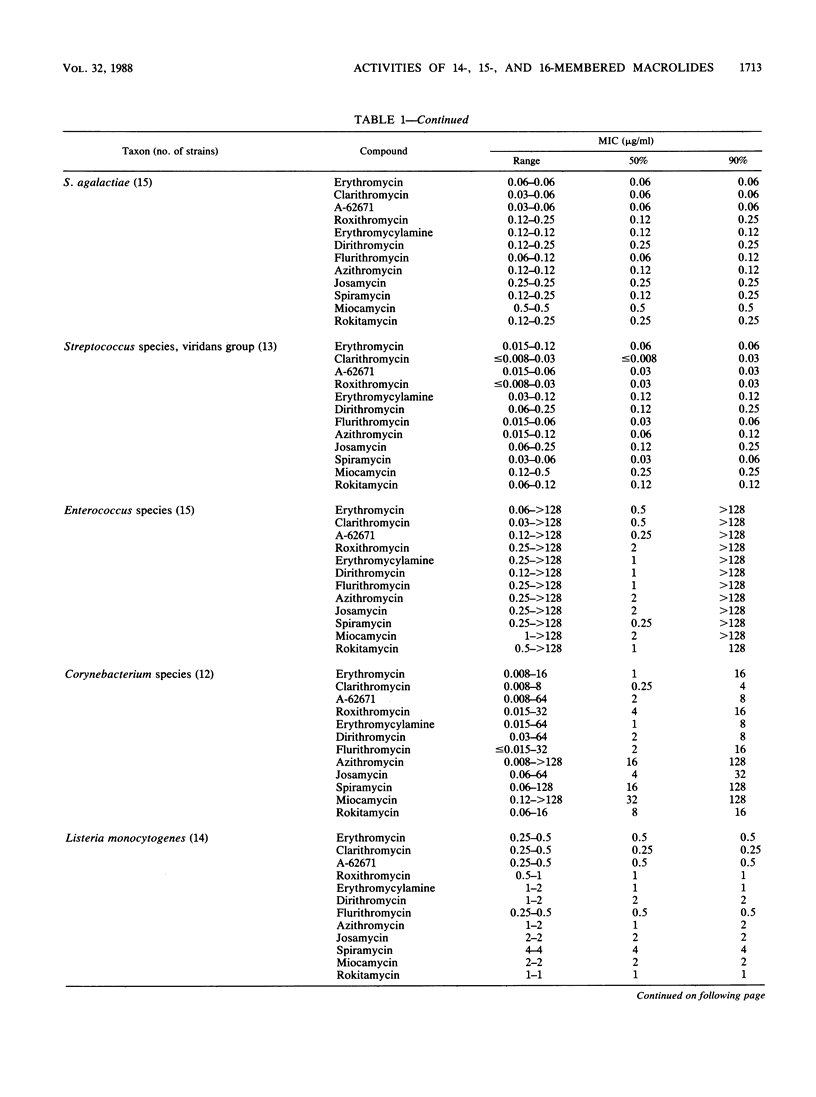
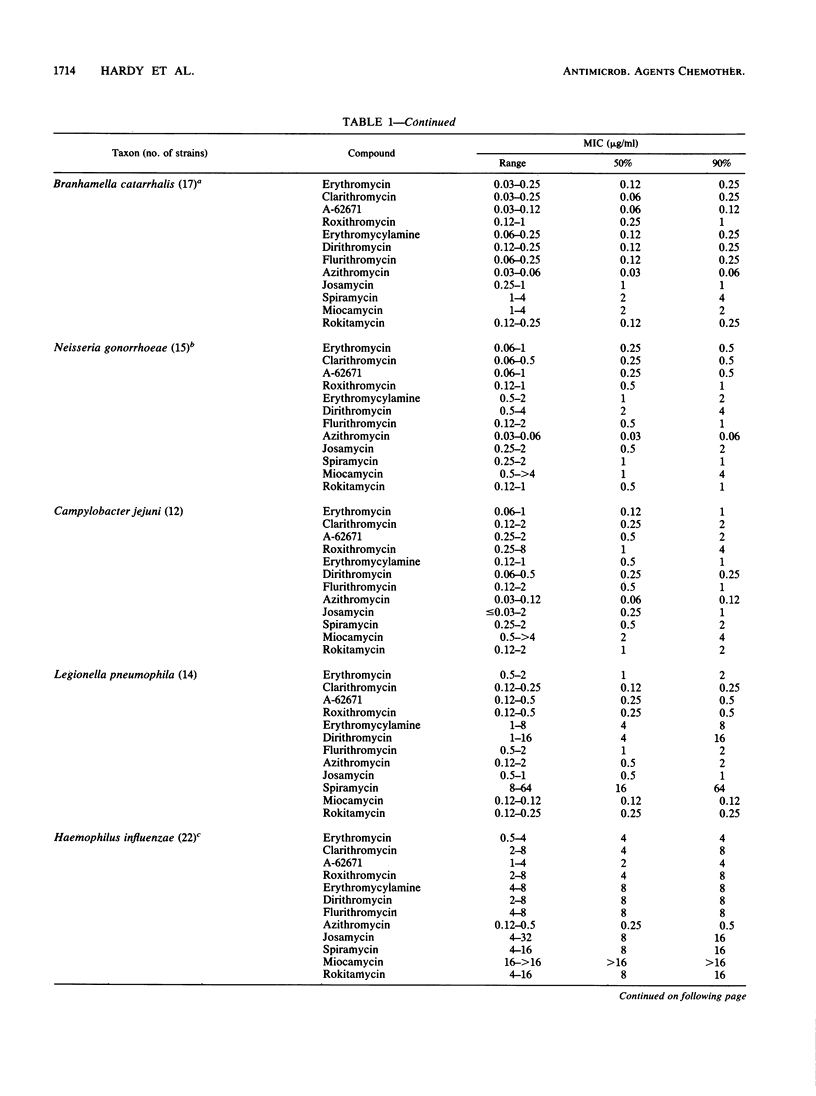
The in vitro activities of several 14-, 15- and 16-membered macrolides were compared with that of erythromycin. In general, 14-membered macrolides such as erythromycin, clarithromycin, and flurithromycin were more active against streptococci and Bordetella pertussis than was the 15-membered macrolide azithromycin, which was more active than 16-membered macrolides such as miocamycin and rokitamycin. Clarithromycin was the most active compound against Streptococcus pyogenes, pneumococci, Listeria monocytogenes, and Corynebacterium species. Legionella pneumophila was most susceptible to miocamycin, clarithromycin, and rokitamycin. Branhamella catarrhalis, Neisseria gonorrhoeae, and Haemophilus influenzae were most susceptible to azithromycin. Azithromycin and dirithromycin were the most active compounds against Campylobacter jejuni. MICs of 16-membered macrolides for strains expressing inducible-type resistance to erythromycin were less than or equal to 1 microgram/ml, whereas none of the compounds had activity against strains expressing constitutive-type resistance. The MICs of roxithromycin, miocamycin, rokitamycin, and josamycin increased in the presence of human serum, whereas MICs of the other compounds either were unchanged or decreased.
Full text
PDF
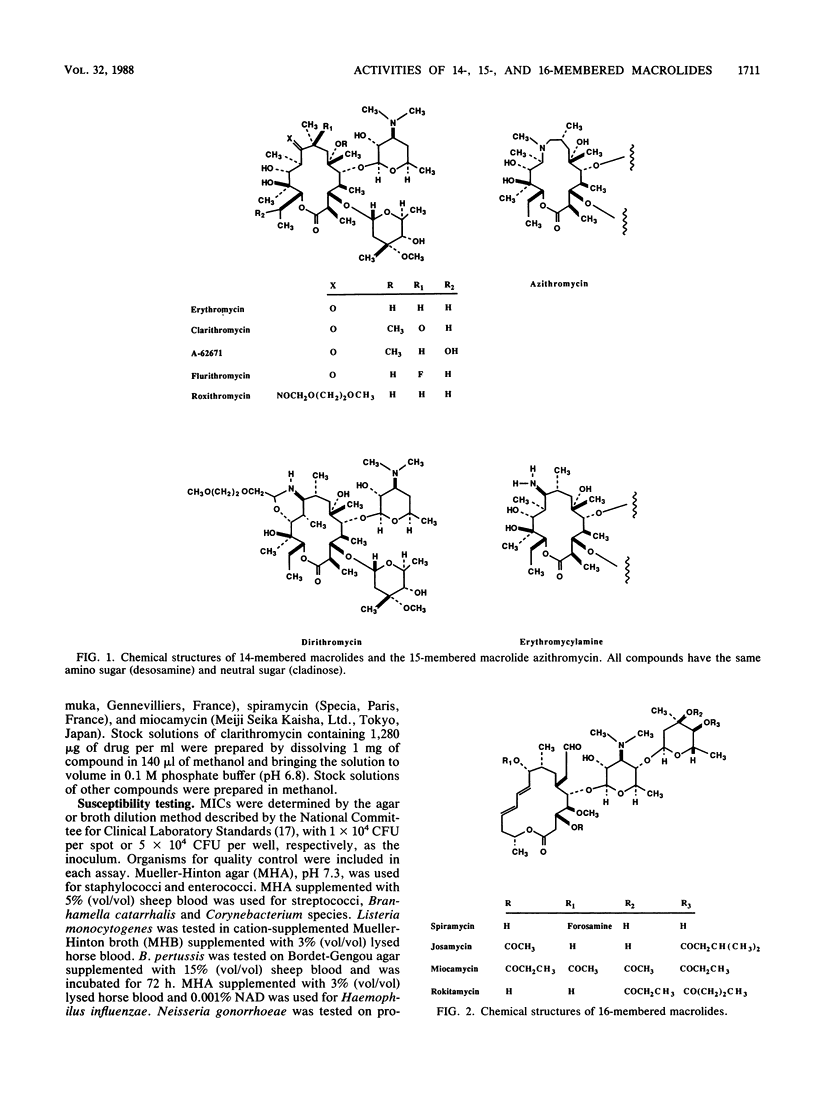
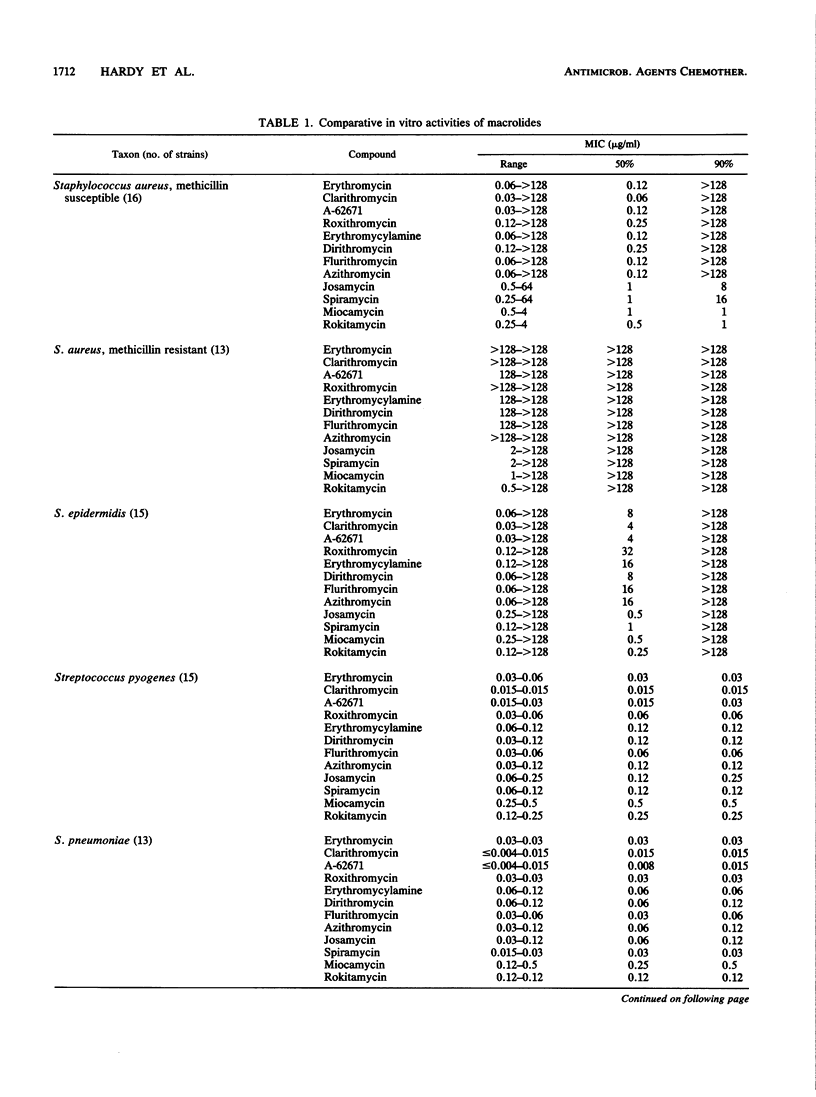


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews J. M., Ashby J. P., Wise R. Factors affecting the in-vitro activity of roxithromycin. J Antimicrob Chemother. 1987 Nov;20 (Suppl B):31–37. doi: 10.1093/jac/20.suppl_b.31. [DOI] [PubMed] [Google Scholar]
- Barker J., Farrell I. D. The effect of charcoal on MICs for Legionella. J Antimicrob Chemother. 1986 Jan;17(1):127–127. doi: 10.1093/jac/17.1.127. [DOI] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N., Thornsberry C. In vitro activities of azithromycin (CP 62,993), clarithromycin (A-56268; TE-031), erythromycin, roxithromycin, and clindamycin. Antimicrob Agents Chemother. 1988 May;32(5):752–754. doi: 10.1128/aac.32.5.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein N., Roudier C., Fleurette J. Determination of the activity on Legionella of eight macrolides and related agents by comparative testing on three media. J Antimicrob Chemother. 1985 Jan;15(1):17–22. doi: 10.1093/jac/15.1.17. [DOI] [PubMed] [Google Scholar]
- Chantot J. F., Bryskier A., Gasc J. C. Antibacterial activity of roxithromycin: a laboratory evaluation. J Antibiot (Tokyo) 1986 May;39(5):660–668. doi: 10.7164/antibiotics.39.660. [DOI] [PubMed] [Google Scholar]
- Dette G. A., Knothe H., Koulen G. Comparative in vitro activity, serum binding and binding activity interactions of the macrolides A-56268, RU-28965, erythromycin and josamycin. Drugs Exp Clin Res. 1987;13(9):567–576. [PubMed] [Google Scholar]
- Fallon R. J., Brown W. M. In-vitro sensitivity of legionellas, meningococci and mycoplasmas to ciprofloxacin and enoxacin. J Antimicrob Chemother. 1985 Jun;15(6):787–789. doi: 10.1093/jac/15.6.787. [DOI] [PubMed] [Google Scholar]
- Fernandes P. B., Bailer R., Swanson R., Hanson C. W., McDonald E., Ramer N., Hardy D., Shipkowitz N., Bower R. R., Gade E. In vitro and in vivo evaluation of A-56268 (TE-031), a new macrolide. Antimicrob Agents Chemother. 1986 Dec;30(6):865–873. doi: 10.1128/aac.30.6.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L. The antimicrobial activity of A-56268 (TE-031) and roxithromycin (RU965) against Legionella using broth microdilution method. J Antimicrob Chemother. 1987 Jun;19(6):841–842. doi: 10.1093/jac/19.6.841. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. H., Redding J. S., Maher L. A., Howell A. W. Improved medium for antimicrobial susceptibility testing of Haemophilus influenzae. J Clin Microbiol. 1987 Nov;25(11):2105–2113. doi: 10.1128/jcm.25.11.2105-2113.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaharajo K., Sekizawa Y., Inoue M. In vitro and in vivo antibacterial activity of 9,3"-Di-o-acetyl midecamycin (Mom), a new macrolide antibiotic. J Antibiot (Tokyo) 1981 Apr;34(4):436–442. doi: 10.7164/antibiotics.34.436. [DOI] [PubMed] [Google Scholar]
- LeBlanc D. J., Inamine J. M., Lee L. N. Broad geographical distribution of homologous erythromycin, kanamycin, and streptomycin resistance determinants among group D streptococci of human and animal origin. Antimicrob Agents Chemother. 1986 Apr;29(4):549–555. doi: 10.1128/aac.29.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmborg A. S. The renaissance of erythromycin. J Antimicrob Chemother. 1986 Sep;18(3):293–296. doi: 10.1093/jac/18.3.293. [DOI] [PubMed] [Google Scholar]
- Morimoto S., Takahashi Y., Watanabe Y., Omura S. Chemical modification of erythromycins. I. Synthesis and antibacterial activity of 6-O-methylerythromycins A. J Antibiot (Tokyo) 1984 Feb;37(2):187–189. doi: 10.7164/antibiotics.37.187. [DOI] [PubMed] [Google Scholar]
- Nitta K., Yano K., Miyamoto F., Hasegawa Y., Sato T. A new antibiotic, josamycin. II. Biological studies. J Antibiot (Tokyo) 1967 Jul;20(3):181–187. [PubMed] [Google Scholar]
- Omura S., Sano H., Sunazuka T. Structure activity relationships of spiramycins. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):1–11. doi: 10.1093/jac/16.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- Osono T., Oka Y., Watanabe S., Okami Y., Umezawa H. A new antibiotic, josamyicn. I. Isolation and physico-chemical characteristics. J Antibiot (Tokyo) 1967 Jul;20(3):174–180. [PubMed] [Google Scholar]
- Retsema J., Girard A., Schelkly W., Manousos M., Anderson M., Bright G., Borovoy R., Brennan L., Mason R. Spectrum and mode of action of azithromycin (CP-62,993), a new 15-membered-ring macrolide with improved potency against gram-negative organisms. Antimicrob Agents Chemother. 1987 Dec;31(12):1939–1947. doi: 10.1128/aac.31.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara H., Okekawa O., Fujiwara T., Otani M., Omura S. Acyl derivatives of 16-membered macrolides. I. Synthesis and biological properties of 3"-O-propionylleucomycin A5 (TMS-19-Q). J Antibiot (Tokyo) 1981 Aug;34(8):1001–1010. doi: 10.7164/antibiotics.34.1001. [DOI] [PubMed] [Google Scholar]
- Strausbaugh L. J., Dilworth J. A., Gwaltney J. M., Jr, Sande M. A. In vitro susceptibility studies with josamycin and erythromycin. Antimicrob Agents Chemother. 1976 Mar;9(3):546–548. doi: 10.1128/aac.9.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano L., Fioriello G., Spagnoli R., Cappelletti L., Zanuso G. New fluorinated erythromycins obtained by mutasynthesis. J Antibiot (Tokyo) 1983 Nov;36(11):1439–1450. doi: 10.7164/antibiotics.36.1439. [DOI] [PubMed] [Google Scholar]


