Abstract
Textural properties and functional morphology of the hip bone cancellous network of Oreopithecus bambolii, a 9- to 7-million-year-old Late Miocene hominoid from Italy, provide insights into the postural and locomotor behavior of this fossil ape. Digital image processing of calibrated hip bone radiographs reveals the occurrence of trabecular features, which, in humans and fossil hominids, are related to vertical support of the body weight, i.e., to bipedality.
The recent claim for a significant bipedal component in the locomotor repertoire of Oreopithecus bambolii (1, 2), an endemic hominoid known from the Late Miocene [9- to 7-million-year-old (Ma)] Tyrrhenian island (3, 4), revives the still open debate on the origin(s) and evolution of hominid bipedality (5). Evidence derived from the structural analysis of the Oreopithecus iliac cancellous network supports this claim.
In the vertebrate skeletal system, the hip is a key bone. In clinogrades and most pronogrades, it transmits propulsive force from the hind limbs to the trunk and part of the trunk weight to the hind limbs (≈50–60% of the weight carried by obligatory bipeds), whereas in bipeds, it shifts the entire body weight from the lower lumbar vertebrae, the sacrum, and the sacroiliac joints through the ilium to the acetabulum and onto the head and neck of the femur (6).
Bone is a self-optimizing material displaying the capacity for nondestructive energy dissipation and the influence of strain rate on strength and stiffness (7). Consisting of a cancellous network covered by a thin cortical shell, the hip bone behaves like a “sandwich” construction, a three-dimensional structure combining high strength with a relatively low density. In a “sandwich” construction, the bulk of the load is carried by a thin shell of high-modulus material (the cortical bone), whereas the low-weight core material (the cancellous bone) acts as a spacer in separating the outer sheets of compact bone and, more importantly, in resisting and dissipating shear stresses (8). Its fabric (architecture) is shaped primarily by the site-specific magnitude and direction of the locomotion-related peak strains habitually imposed on the growing bone. Low-density, open-cell, rod-like structures occur in regions of low strain (where low-density trajectories follow minimum stresses), whereas high-density, closed-cell, plate-like structures develop in regions of higher strains (where high-density trajectories follow maximum stresses) (9). On the whole, more than 80% of the variance in cancellous bone biomechanical behavior can be explained by measures of site-specific density and textural orientation (10).
Among the extant mammals, primates display a great diversity of postural and locomotor behaviors because of their adaptive ability to exploit a full range of arboreal and terrestrial substrates (11). Because cancellous bone architecture and mechanics are intimately related (12, 13), similar variation also is found in the structural organization, patterning, and degree of textural anisotropy of the iliac cancellous network, where rather specific, locomotion-related architectural patterns can be recognized.
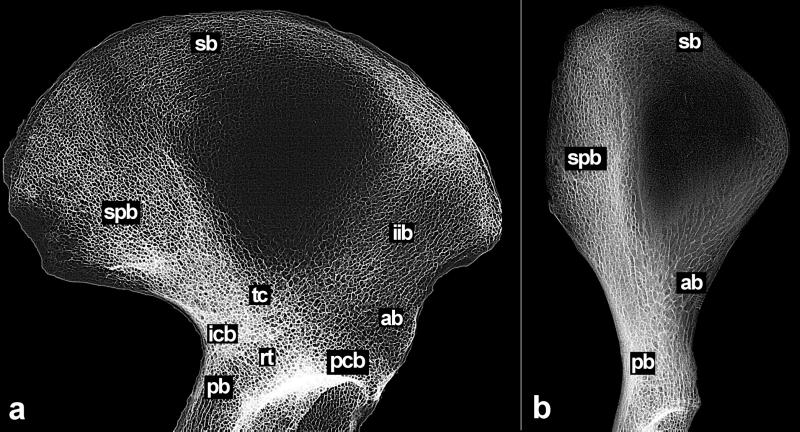
In the human iliac blade (Fig. 1a), the sacropubic (spb) and the ilioischial (iib) trabecular bundles absorb and distribute the loads generated during striding gait (14). These bundles cross over the acetabulum, forming a high-density trabecular network, or chiasma (tc), transversally located between the sciatic notch and the anterior inferior iliac spine (15, 16). Along with the adoption of bipedal gait as an obligatory locomotion mode, in humans this pattern is established early in childhood through progressive, site-specific strengthening of the trabeculae, starting from a poorly differentiated cancellous network. As biomechanical strains increase in frequency and magnitude, trabeculae undergo functional thickening and patterning (especially along the sacropubic bundle running from the auricular surface and the posterior superior and inferior iliac spines toward the trabecular chiasma), and the degree of anisotropy of the network as a whole progressively increases. Because of differences in gross pelvic morphology and the magnitude and direction of the habitual, locomotion-related loads applied to the ilium, this pattern is not found in extant apes (Fig. 1b).
Figure 1.
Iliac trabecular architecture in Homo sapiens (SCR 252) (a) and Hylobates syndactylus (AIZIU 1726) (b) (not to scale). ab, anterior; sb, superior; pb, posterior; pcb, pericotyloid; icb, iliocotyloid; spb, sacropubic bundle; and iib, ilioischial bundle; rt, radial trabeculae; tc, trabecular crossing between the spbs and the iibs. Major gait-related features in the trabecular system of human ilium (a) include: a distinctive iib, a strong, undivided spb, and a diagonal full crossing (tc) of these bundles over the acetabulum. In H. syndactylus (b), as well as in all extant apes, a true iib is absent because of the lack of trabecular structural organization, resulting in a honeycomb-like cancellous network. The poorly structured inner bundles do not form a full crossing, but only partially flow into a slightly higher-density confluence of trabeculae located well high above the acetabular upper rim.
Two O. bambolii hip bones suitable for structural analysis of the iliac cancellous network are available in the fossil record: BAC 76, a fragmentary blade and corpus of a right ilium, and IGF 11778, an articulated skeleton of a young adult preserving the incomplete right and left ilia, the left ischium, pubis, and the sacrum.
Preliminary radiographic and tomographic investigation of BAC 76 proved no specific trabecular features. Despite its squashed external aspect, the internal morphology of IGF 11778 iliac bones showed trabecular structure preserved well enough to allow architectural analysis and biomechanical interpretation.
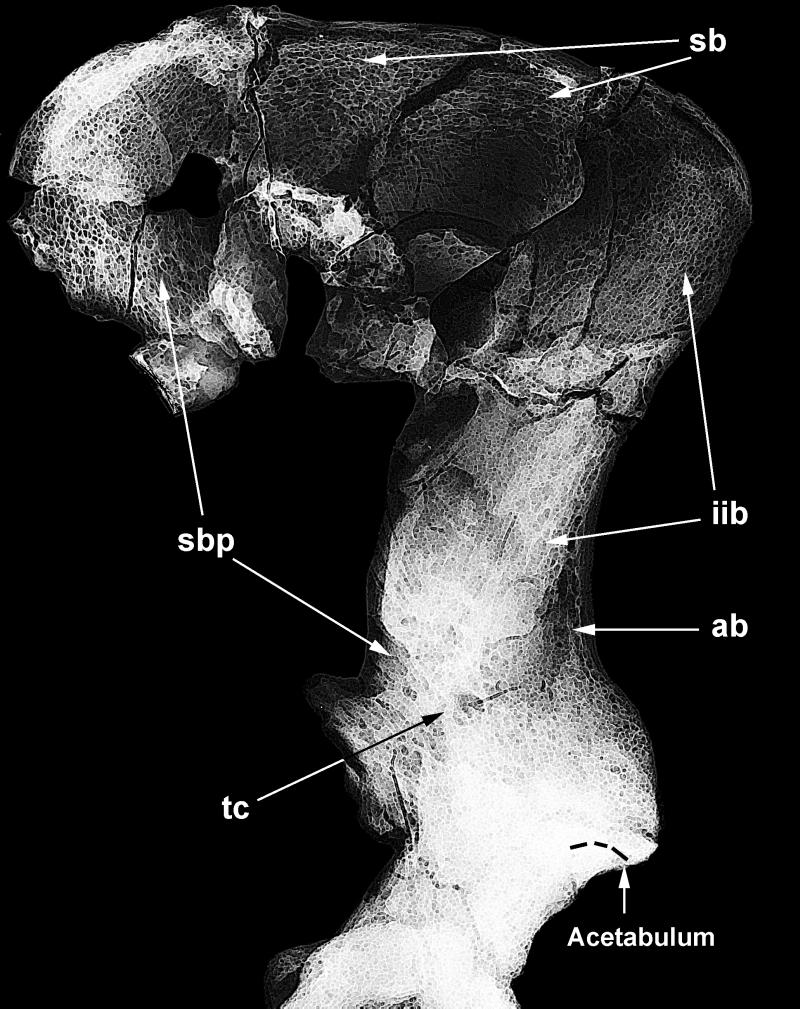
To enhance the quality of the cancellous textural features, the investigation was carried out by means of advanced digital image processing techniques applied to a set of calibrated x-ray films.¶ A virtually complete Oreopithecus hip bone has been reconstructed by digital superimposition and textural alignment of the mirrored right iliac blade to the left specimen (Fig. 2).
Figure 2.
Electronically enhanced trabecular architecture of the O. bambolii ilium (left and mirrored-right IGF 11778 specimens digitally overlapped). See Fig. 1 caption for explanation of the bundle labels.
In the enhanced digital image, the Oreopithecus trabecular bundles appear well structured. They distinctly run as a frame along the free margins of the blade as well as across the iliac body. Similar to humans and fossil hominids (17, 18), the Oreopithecus inner bundles (spb and iib) are quite distinct from the trabecular frame, whereas they fade in both monkeys and apes. In particular, the superior and the posterior marginal bundles (Fig. 3a) are proportionally thicker than in extant apes of any size. In this feature they resemble the human condition. Furthermore, the posterosuperior rim of the Oreopithecus blade (Fig. 3a) displays a higher density area, a feature occasionally observed in humans but absent in orangutans and chimpanzees.
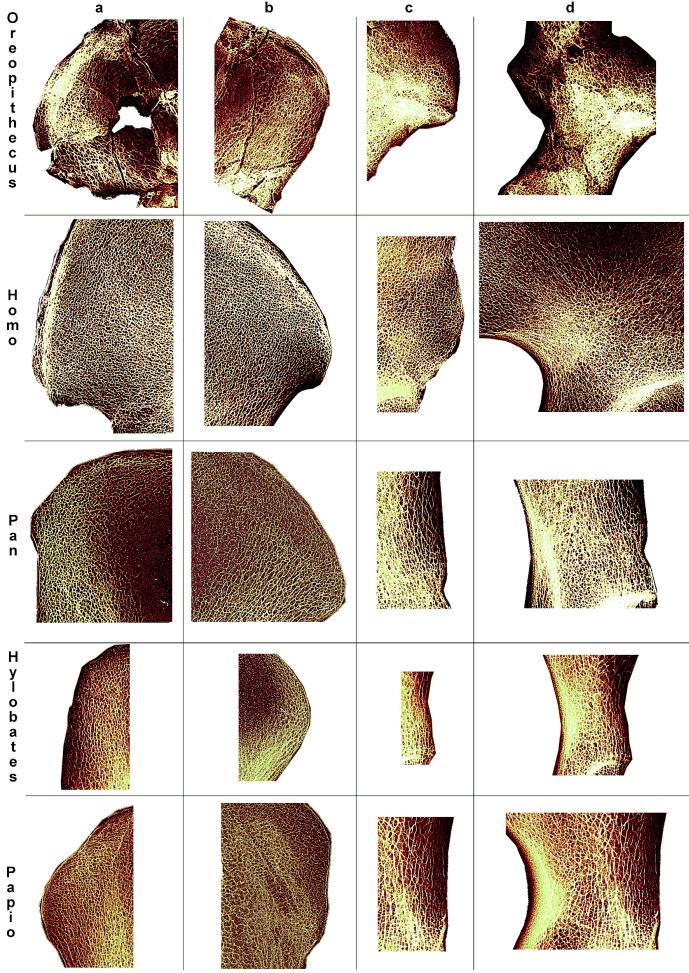
Figure 3.
Comparative site-specific structural morphology of the hip bone in Oreopithecus (IGF 11778), Homo (SCR 352), Pan (PVA 2706), Hylobates (AIZIU 1726), and Papio (AIZIU PAL 109). Iliac blade posterosuperior margin (a), anterosuperior margin (b), anteroinferior margin (c), and supraacetabular area (d) are shown. Because specimens are not reproduced to scale, the sizes of the trabecular mesh are not directly comparable.
Unlike monkeys and lesser apes, the Oreopithecus inner patterning is distinctly structured (Fig. 2). A spb bundle is traceable from the posterosuperior portion of the blade toward the iliac body and the upper acetabular rim. As in hominids, plate-like structures apparently occur at its root, but the trabeculae become thinner running inferiorly and anteriorly. The superior portion of the Oreopithecus iib bundle is also relatively structured and differentiated compared with the honeycomb-like pattern shown by apes, but is less than in humans (Fig. 3b).
As in humans, the Oreopithecus hip bone external morphology is characterized by the occurrence of a well-developed anteroinferior iliac spine (1). At this site, the trabecular structure resembles that of Homo (Fig. 3c).
The highest-density site of the Oreopithecus iliac cancellous network corresponds to the supraacetabular region (the trabecular chiasma), where substantial portions of the spb and the iib bundles cross (Fig. 3d). At this site, the Oreopithecus architecture is less developed than in humans and australopithecines (18), but a comparative degree of development as seen in this fossil hominoid is not observed for any other ape, except among gorillas (likely caused by the combined effect of body weight and allometry).
Overall, the textural features of the Oreopithecus iliac cancellous network testify that the strength and direction of the positional/locomotor-related peak strains that acted upon this fossil hominoid not only differed from those observed in any nonhuman primate (including lesser and great apes), but were compatible with the biomechanical requirements for habitual upper body weight support and transmission to the lower limbs. Accordingly, it is not surprising that the gross anatomy of its pelvis shows some other key morphological features related to bipedal locomotion, including an extraordinary long, human-like ischial spine, a well-developed anteroinferior iliac spine, a short pubic symphysis, and a short ischium (1).
In conclusion, this unique combination in the primate fossil record of external morphological and internal trabecular features of the pelvis is fully compatible with the proposition that the postural and locomotor behavior of this Late Miocene hominoid included habitual bipedality (1, 2).
Acknowledgments
We thank B. Engesser, D. Torre, and E. Cioppi for access to the original specimens. For their comments, suggestions, and criticism on a previous draft of this paper we are extremely grateful to L. Aiello, P. Andrews, C. O. Lovejoy, P. O’Higgins, D. Pilbeam, P. Schmid, J. Schwartz, D. Weaver, and M. H. Wolpoff. We thank the Anthropologisches Institut of the Zurich-Irchel University, the Transvaal Museum, the University of the Witwatersrand, the National Museums of Kenya, the Tervuren Zoo, the Museo Civico di Zoologia of Rome, the Museo di Storia Naturale of the University of Pavia, and the National Prehistoric Ethnographic “L. Pigorini” Museum for access to the osteological material in their care. The Hospital “C. Forlanini” and the “Centro Diagnostico L. Da Vinci” provided technical support for radiographs. Research was funded by the L. S. B. Leakey Foundation and the University of Florence (L.R.), CICYT PB97-1173 (S.M.-S.) and the Italian Consiglio Nazionale delle Ricerche “Progelto Finalizzato BeniCulturali” (R.M.).
ABBREVIATIONS
- spb
sacropubic bundle
- iib
ilioischial bundle
- AIZIU
Anthropologisches Institut, Zurich-Irchel University, Switzerland
- BAC
Baccinello Collection, Naturhistorisches Museum, Basel, Switzerland
- IGF
Museo di Storia Naturale, Sezione di Geologia e Paleontologia, University of Forence, Italy
- PVA
Museo di Storia Naturale, University of Pavia, Italy
- SCR
Isola Sacra Osteodental Collection, Museo Nazionale Preistorico Etnografico “L. Pigorini,” Rome, Italy
Footnotes
Calibrated original x-ray films were transferred into a numerical format with an Agfa ARCUS 2 transparency scanner in a resolution of 600 dots per inch (24 bits). The software used for digital image processing was a compound of three digital image processing packages: nih image 1.62, graftek optilab pro 2.5, and specific deconvolution routines (19). For an exhaustive description of digital image processing procedures, the reader is addressed to refs. 18 and 20. The whole body of original and processed digital images is available at the Section of Anthropology of the National Prehistoric Ethnographic “L. Pigorini” Museum (Rome). The comparative radiographic sample includes 196 infant, juvenile, and adult humans and 100 specimens of extant primates representing prosimians (Propithecus, Perodicticus), New World monkeys (Lagothrix, Alouatta), Old World monkeys (Colobus, Presbytis, Theropithecus, Papio, Macaca, Cercopithecus), lesser (Hylobates), and great apes (Pongo, Gorilla, Pan). Besides these primate taxa, for comparative purposes we also have investigated the iliac cancellous patterning in a number of nonprimate mammals (marsupials, sloths, rodents, carnivores, artiodactyls). The entire set of elaborated images is collected into a CD-ROM (in preparation) within the monographic series Digital Archives of Human Paleobiology.
References
- 1.Köhler M, Moyà-Solà S. Proc Natl Acad Sci USA. 1997;94:11747–11750. doi: 10.1073/pnas.94.21.11747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moyà-Solà S, Köhler M, Rook L. Proc Natl Acad Sci USA. 1999;96:313–317. doi: 10.1073/pnas.96.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moyá-Solá S, Köhler M. C R Acad Sci Paris. 1996;324:141–148. [Google Scholar]
- 4.Harrison T, Rook L. In: Function, Phylogeny, and Fossils: Miocene Hominoid Origins and Adaptations. Begun D R, Ward C W, Rose M D, editors. New York: Plenum; 1997. pp. 327–362. [Google Scholar]
- 5.Coppens Y, Senut B, editors. Origine(s) de la Bipédie ches les Hominidés. Paris: Centre National de la Recherche Scientifique; 1991. [Google Scholar]
- 6.Reynolds T R. Am J Phys Anthropol. 1988;67:335–349. doi: 10.1002/ajpa.1330670406. [DOI] [PubMed] [Google Scholar]
- 7.Linde F, Nørgaard P, Hvid I, Odgaard A, Søballe K. J Biomech. 1991;24:803–809. doi: 10.1016/0021-9290(91)90305-7. [DOI] [PubMed] [Google Scholar]
- 8.Currey J D. The Mechanical Adaptations of Bones. Princeton: Princeton Univ. Press; 1984. [Google Scholar]
- 9.Gibson L J. J Biomech. 1985;18:317–328. doi: 10.1016/0021-9290(85)90287-8. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein S A, Goulet R, McCubbrey D. Calcif Tissue Int. 1993;53, Suppl. 1:127–133. doi: 10.1007/BF01673421. [DOI] [PubMed] [Google Scholar]
- 11.Fleagle J G. Primate Adaptation and Evolution. San Diego: Academic; 1999. [Google Scholar]
- 12.Odgaard A, Kabel J, van Rietbergen B, Dalstra M, Huiskes R. J Biomech. 1997;30:487–495. doi: 10.1016/s0021-9290(96)00177-7. [DOI] [PubMed] [Google Scholar]
- 13.Luo Z P, An K N. J Math Biol. 1998;36:557–568. doi: 10.1007/s002850050114. [DOI] [PubMed] [Google Scholar]
- 14.Dalstra M, Huiskes R. J Biomech. 1995;28:715–724. doi: 10.1016/0021-9290(94)00125-n. [DOI] [PubMed] [Google Scholar]
- 15.Correnti V. Riv Antropol. 1955;42:289–336. [Google Scholar]
- 16.Dalstra M, Huiskes R, Odgaard A, Van Erning L. J Biomech. 1993;26:523–535. doi: 10.1016/0021-9290(93)90014-6. [DOI] [PubMed] [Google Scholar]
- 17.Macchiarelli R, Galichon V, Bondioli L, Tobias P V. In: Proceedings of the XIII International Congress of Prehistoric and Protohistoric Sciences. Facchini F, Palma di Cesnola A, Piperno M, Peretto C, editors. Vol. 2. Forlì, Italy: ABACO; 1998. pp. 35–41. [Google Scholar]
- 18.Macchiarelli R, Bondioli L, Galichon V, Tobias P V. J Hum Evol. 1999;36:211–232. doi: 10.1006/jhev.1998.0267. [DOI] [PubMed] [Google Scholar]
- 19.Watkins C, Sadun A, Marenka S. Modern Image Processing: Warping, Morphing, and Classical Techniques. Boston: Academic; 1993. [Google Scholar]
- 20.Macchiarelli R, Geusa G, Rossi P F, Salomone F, Sperduti A, Bondioli L. Science and Technology for the Safeguard of Cultural Heritage in the Mediterranean Basin. Vol. 2. Tipolitografia Luxograph, Palermo, Italy: C. N. R.; 1998. pp. 1411–1423. [Google Scholar]