Abstract
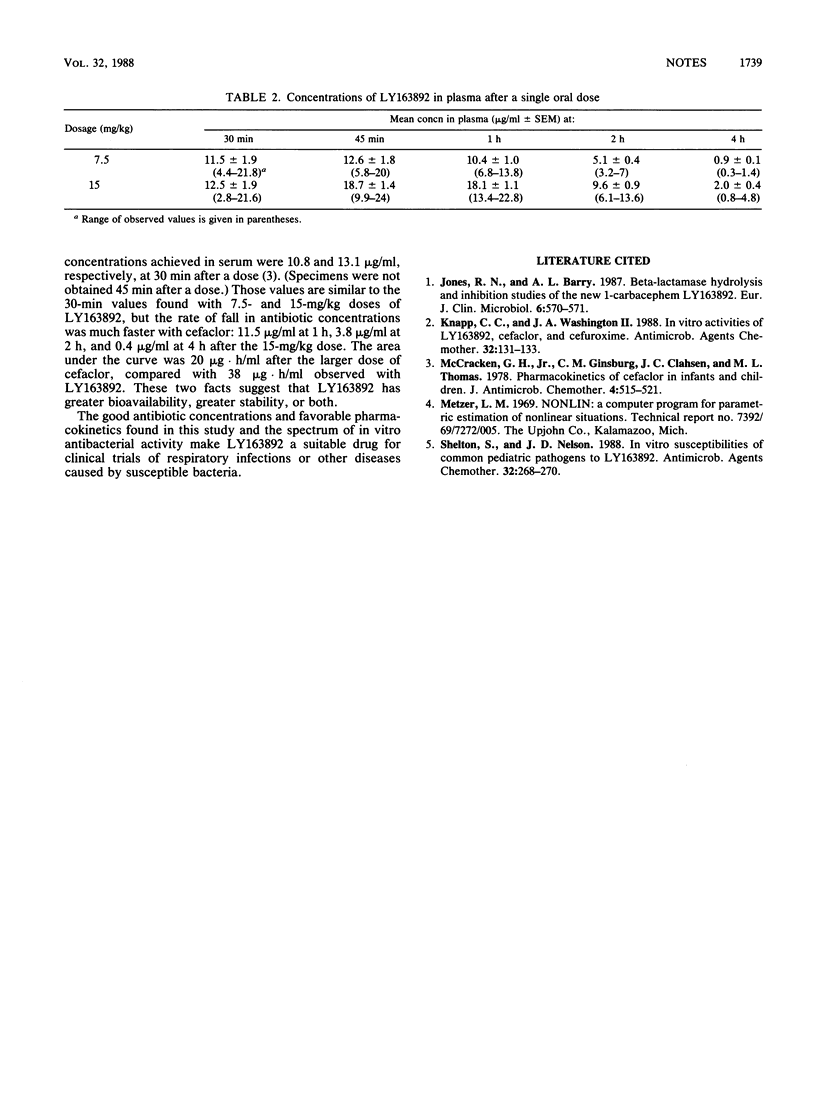
Two dosages (7.5 and 15 mg/kg of body weight) of LY163892 were given to infants and children, and five plasma specimens were collected for antibiotic assay during the ensuing 4 h. Peak concentrations of 12.6 and 18.7 micrograms/ml occurred at 45 min. The mean half-lives were 0.85 and 0.78 h, and the mean areas under the curve were 24.4 and 38.0 micrograms.h/ml.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Jones R. N., Barry A. L. Beta-lactamase hydrolysis and inhibition studies of the new 1-carbacephem LY163892. Eur J Clin Microbiol. 1987 Oct;6(5):570–571. doi: 10.1007/BF02014248. [DOI] [PubMed] [Google Scholar]
- Knapp C. C., Washington J. A., 2nd In vitro activities of LY163892, cefaclor, and cefuroxime. Antimicrob Agents Chemother. 1988 Jan;32(1):131–133. doi: 10.1128/aac.32.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken G. H., Jr, Ginsburg C. M., Clahsen J. C., Thomas M. L. Pharmacokinetics of cefaclor in infants and children. J Antimicrob Chemother. 1978 Nov;4(6):515–521. doi: 10.1093/jac/4.6.515. [DOI] [PubMed] [Google Scholar]
- Shelton S., Nelson J. D. In vitro susceptibilities of common pediatric pathogens to LY163892. Antimicrob Agents Chemother. 1988 Feb;32(2):268–270. doi: 10.1128/aac.32.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]


