Abstract
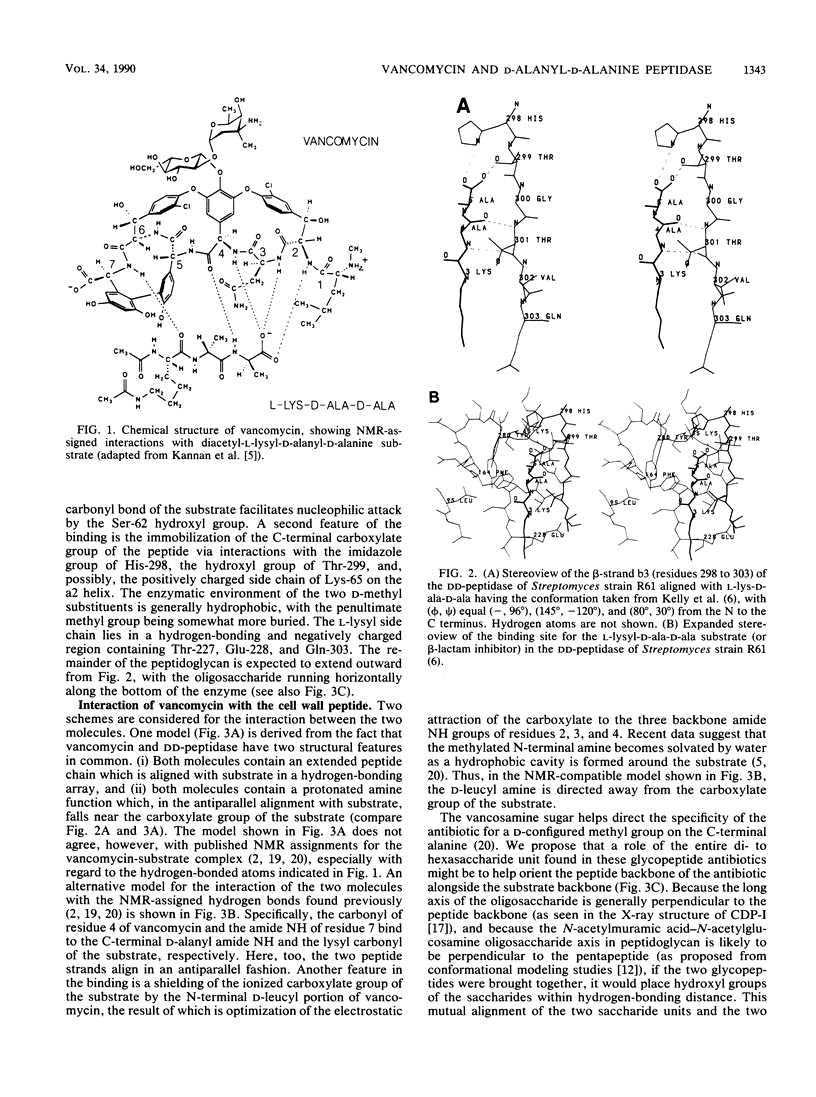
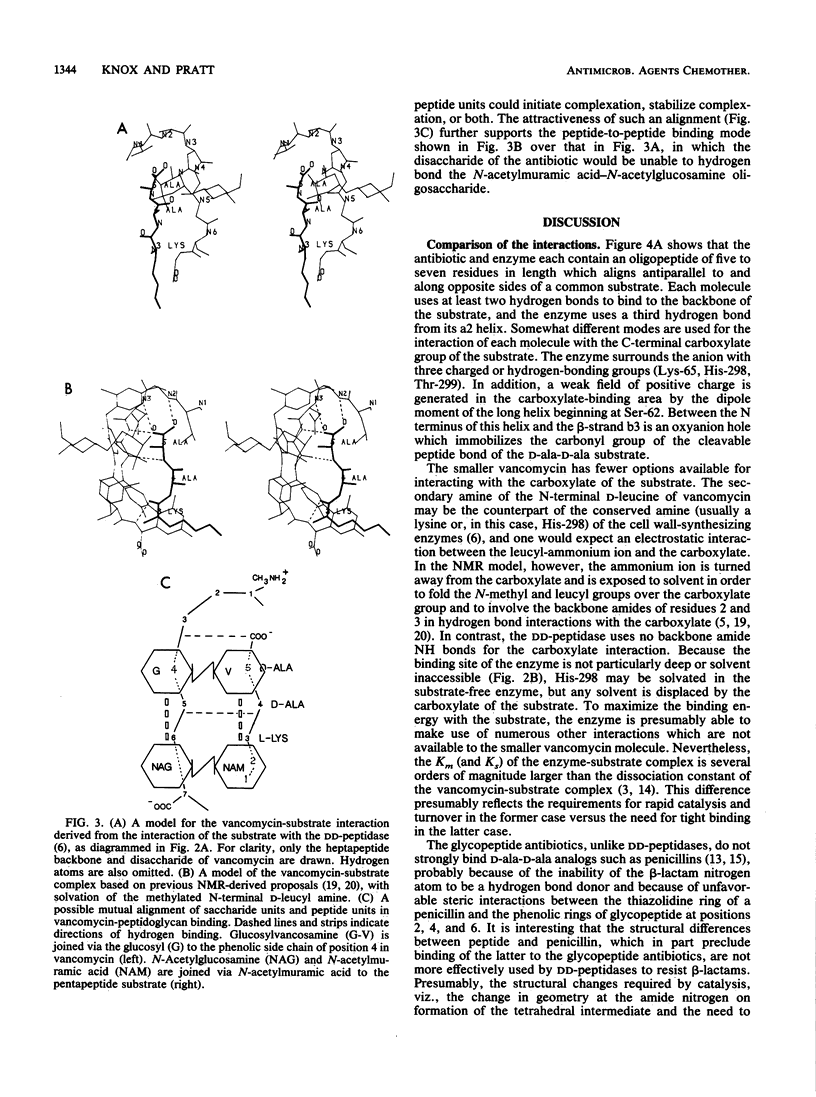
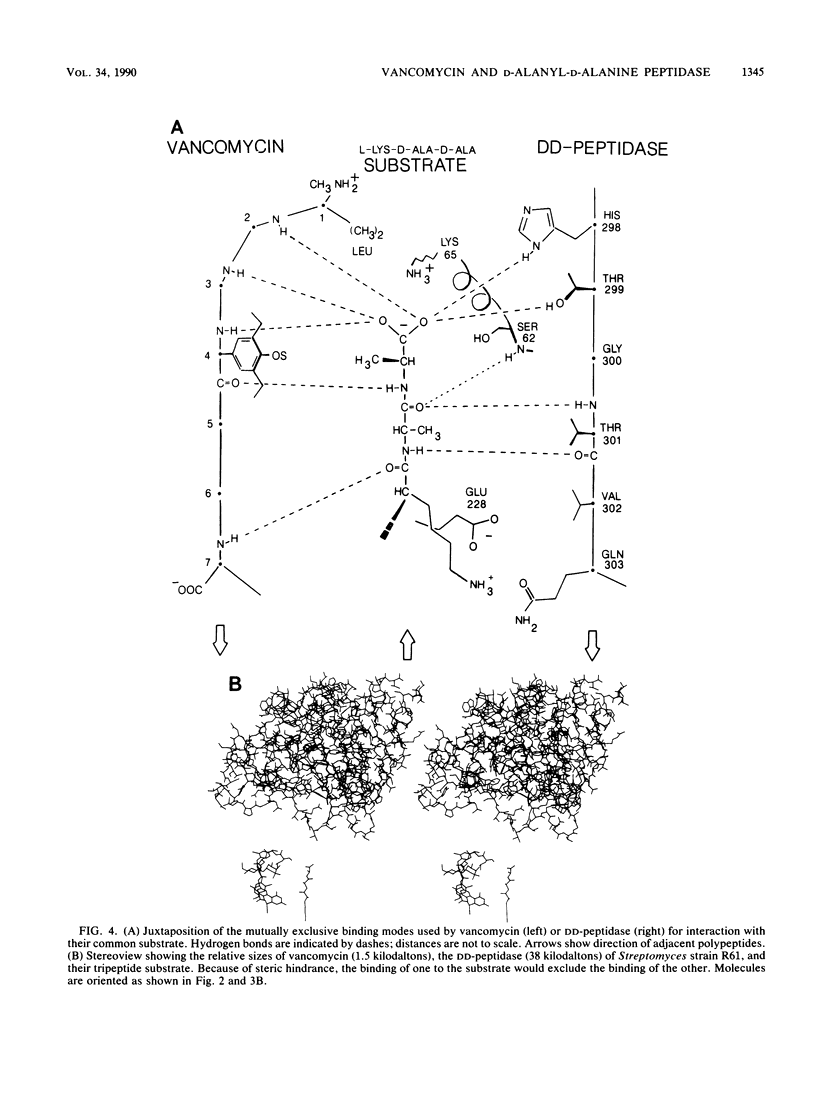
A comparison was made of the binding modes of the bacterial cell wall precursor L-lysyl-D-alanyl-D-alanine to the glycopeptide antibiotic vancomycin and to the D-alanyl-D-alanine-cleaving peptidase of Streptomyces sp. strain R61, a model for cell wall-synthesizing enzymes whose X-ray three-dimensional structure is established. In each of the two pairings (vancomycin with peptide and DD-peptidase with peptide), polypeptide backbones were antiparallel, and the antibiotic or enzyme enveloped the peptide substrate from opposite sides. Hydrogen-bonding groups on the substrate which are involved with the DD-peptidase were shown to be different from the ones reported from nuclear magnetic resonance studies to be involved with vancomycin. Because of steric hindrance, the binding of either molecule to the substrate prevents the binding of the other molecule. Binding to the substrate by a D-alanyl-D-alanine-recognizing protein in a manner similar to that used by the DD-peptidase could explain recent observations of vancomycin resistance, in which a new membrane-associated protein has been detected.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Frère J. M., Joris B. Penicillin-sensitive enzymes in peptidoglycan biosynthesis. Crit Rev Microbiol. 1985;11(4):299–396. doi: 10.3109/10408418409105906. [DOI] [PubMed] [Google Scholar]
- Jones T. A. Diffraction methods for biological macromolecules. Interactive computer graphics: FRODO. Methods Enzymol. 1985;115:157–171. doi: 10.1016/0076-6879(85)15014-7. [DOI] [PubMed] [Google Scholar]
- Kelly J. A., Knox J. R., Zhao H., Frère J. M., Ghaysen J. M. Crystallographic mapping of beta-lactams bound to a D-alanyl-D-alanine peptidase target enzyme. J Mol Biol. 1989 Sep 20;209(2):281–295. doi: 10.1016/0022-2836(89)90277-5. [DOI] [PubMed] [Google Scholar]
- Knox J. R., Liu H. S., Walsh C. T., Zawadzke L. E. D-alanine-D-alanine ligase (ADP) from Salmonella typhimurium. Overproduction, purification, crystallization and preliminary X-ray analysis. J Mol Biol. 1989 Jan 20;205(2):461–463. doi: 10.1016/0022-2836(89)90357-4. [DOI] [PubMed] [Google Scholar]
- Leclercq R., Derlot E., Duval J., Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988 Jul 21;319(3):157–161. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- Neuhaus F. C., Carpenter C. V., Miller J. L., Lee N. M., Gragg M., Stickgold R. A. Enzymatic synthesis of D-alanyl-D-alanine. Control of D-alanine:D-alanine ligase (ADP). Biochemistry. 1969 Dec;8(12):5119–5124. doi: 10.1021/bi00840a066. [DOI] [PubMed] [Google Scholar]
- Nicas T. I., Wu C. Y., Hobbs J. N., Jr, Preston D. A., Allen N. E. Characterization of vancomycin resistance in Enterococcus faecium and Enterococcus faecalis. Antimicrob Agents Chemother. 1989 Jul;33(7):1121–1124. doi: 10.1128/aac.33.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldmixon E. H., Glauser S., Higgins M. L. Two proposed general configurations for bacterial cell wall peptidoglycans shown by space-filling molecular models. Biopolymers. 1974;13(10):2037–2060. doi: 10.1002/bip.1974.360131008. [DOI] [PubMed] [Google Scholar]
- Perkins H. R. Specificity of combination between mucopeptide precursors and vancomycin or ristocetin. Biochem J. 1969 Jan;111(2):195–205. doi: 10.1042/bj1110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popieniek P. H., Pratt R. F. A fluorescent ligand for binding studies with glycopeptide antibiotics of the vancomycin class. Anal Biochem. 1987 Aug 15;165(1):108–113. doi: 10.1016/0003-2697(87)90207-7. [DOI] [PubMed] [Google Scholar]
- Sheldrick G. M., Jones P. G., Kennard O., Williams D. H., Smith G. A. Structure of vancomycin and its complex with acetyl-D-alanyl-D-alanine. Nature. 1978 Jan 19;271(5642):223–225. doi: 10.1038/271223a0. [DOI] [PubMed] [Google Scholar]
- Shlaes D. M., Bouvet A., Devine C., Shlaes J. H., al-Obeid S., Williamson R. Inducible, transferable resistance to vancomycin in Enterococcus faecalis A256. Antimicrob Agents Chemother. 1989 Feb;33(2):198–203. doi: 10.1128/aac.33.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. H., Waltho J. P. Molecular basis of the activity of antibiotics of the vancomycin group. Biochem Pharmacol. 1988 Jan 1;37(1):133–141. doi: 10.1016/0006-2952(88)90765-4. [DOI] [PubMed] [Google Scholar]
- Williamson R., Al-Obeid S., Shlaes J. H., Goldstein F. W., Shlaes D. M. Inducible resistance to vancomycin in Enterococcus faecium D366. J Infect Dis. 1989 Jun;159(6):1095–1104. doi: 10.1093/infdis/159.6.1095. [DOI] [PubMed] [Google Scholar]
- al-Obeid S., Collatz E., Gutmann L. Mechanism of resistance to vancomycin in Enterococcus faecium D366 and Enterococcus faecalis A256. Antimicrob Agents Chemother. 1990 Feb;34(2):252–256. doi: 10.1128/aac.34.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]



