Abstract
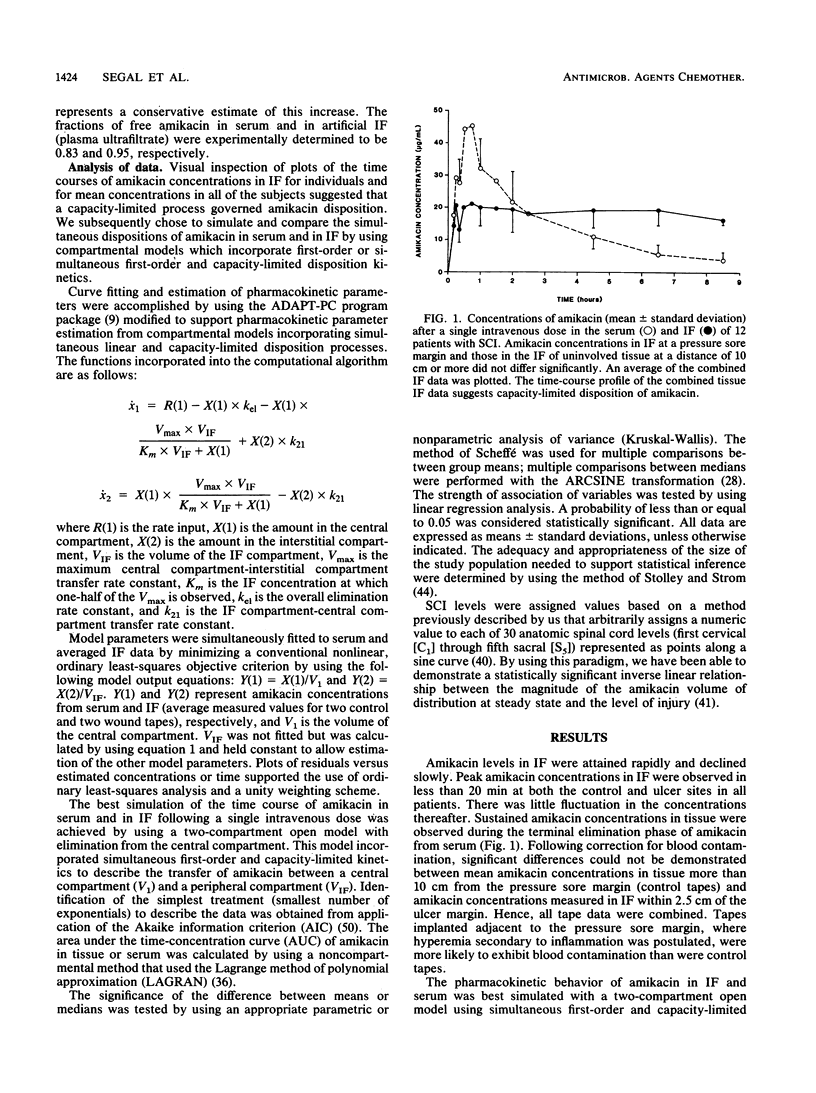
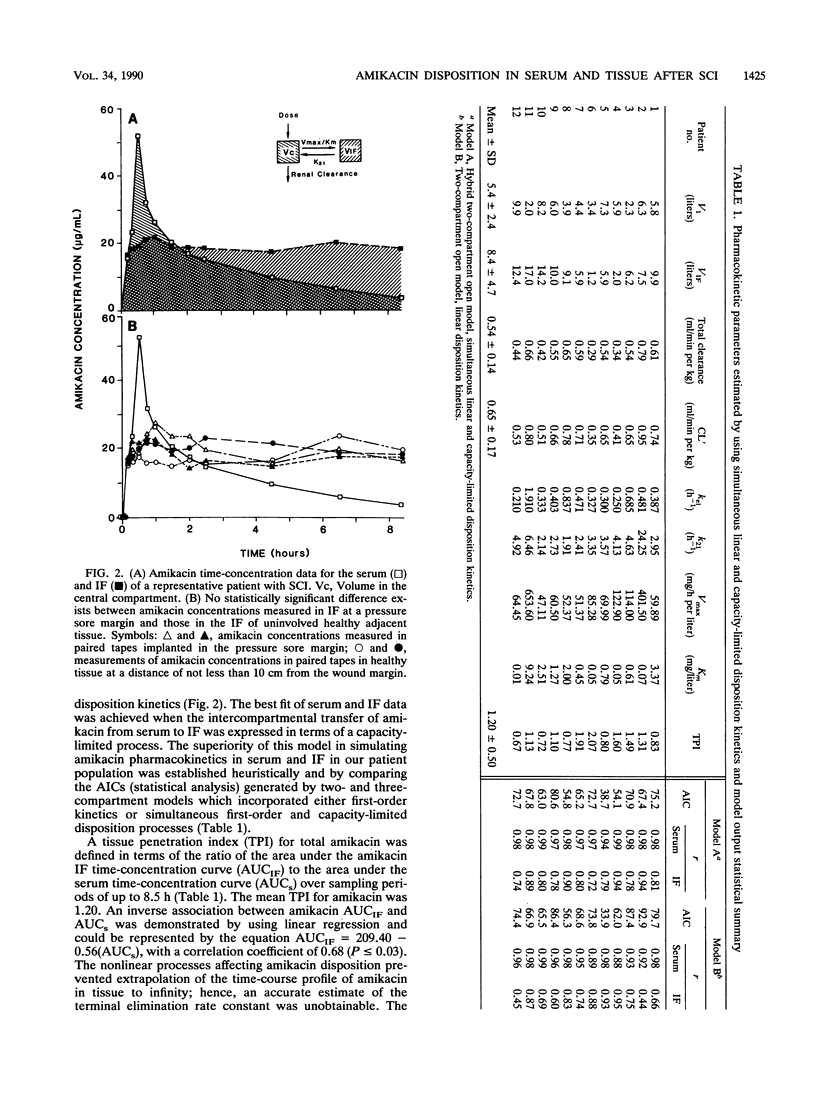
Pressure sores are a common occurrence in immobilized patients. They increase morbidity and mortality and impede rehabilitation. Antibiotics are routinely used to assist in effecting a cure when infection is present. Nevertheless, for patients with spinal cord injuries (SCI), strategies for effective therapy with antibiotics based on measurement of concentrations in tissue and pharmacokinetic behavior in extravascular spaces do not exist. By analyzing the concentration-time profile and protein binding of amikacin in the interstitial fluid (IF) in contact with pressure sores, we found that the disposition of amikacin in the tissue contiguous with pressure sores appears to be governed by simultaneous first-order and capacity-limited pharmacokinetic behavior. Amikacin disposition in IF proceeded without a simple relationship to amikacin concentrations in serum, and the time course in IF was not accurately simulated by linear models of amikacin pharmacokinetic behavior. Total amikacin clearance estimated from a pharmacokinetic model using simultaneous first-order and nonlinear intercompartmental transfer of amikacin was not significantly different from clearance calculated by us in a prior study of amikacin pharmacokinetic behavior in patients with SCI. In patients with SCI, optimal use of amikacin in the treatment of infected pressure sores is contingent upon accurate characterization of the pharmacokinetic behavior of this aminoglycoside in serum and in the IF in contact with these lesions. Only methods which quantitate amikacin concentration and protein binding in IF and incorporate a model that can simultaneously simulate nonlinear and linear disposition processes should be relied upon to influence therapeutic decision making.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anniko M., Berggren D., Holm S. Tissue binding kinetics of tobramycin. An experimental study in the mouse inner ear in vitro. Acta Otolaryngol. 1988 Jan-Feb;105(1-2):120–125. doi: 10.3109/00016488809119454. [DOI] [PubMed] [Google Scholar]
- Bagley D. H., Mac Lowry J., Beazley R. M., Gorschboth C., Ketcham A. S. Antibiotic concentration in human wound fluid after intravenous administration. Ann Surg. 1978 Aug;188(2):202–208. doi: 10.1097/00000658-197808000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S. A., Barza M., Haher J., Bell D., Waintraub S., Burtyk M. L., Kane A. Penetration of antibiotics into decubitus ulcers. J Antimicrob Chemother. 1981 Feb;7(2):193–195. doi: 10.1093/jac/7.2.193. [DOI] [PubMed] [Google Scholar]
- Bryan C. S., Dew C. E., Reynolds K. L. Bacteremia associated with decubitus ulcers. Arch Intern Med. 1983 Nov;143(11):2093–2095. [PubMed] [Google Scholar]
- Chisholm G. D., Waterworth P. M., Calnan J. S., Garrod L. P. Concentration of antibacterial agents in interstitial tissue fluid. Br Med J. 1973 Mar 10;1(5853):569–573. doi: 10.1136/bmj.1.5853.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull J. G., Smith O. H. A preliminary note on demographic and personality correlates of decubitus ulcer incidence. J Psychol. 1973 Nov;85(2D):225–227. doi: 10.1080/00223980.1973.9915650. [DOI] [PubMed] [Google Scholar]
- D'Argenio D. Z., Schumitzky A. A program package for simulation and parameter estimation in pharmacokinetic systems. Comput Programs Biomed. 1979 Mar;9(2):115–134. doi: 10.1016/0010-468x(79)90025-4. [DOI] [PubMed] [Google Scholar]
- Dan M., Halkin H., Rubinstein E. Interstitial fluid concentrations of aminoglycosides. J Antimicrob Chemother. 1981 May;7(5):551–558. doi: 10.1093/jac/7.5.551. [DOI] [PubMed] [Google Scholar]
- Eltorai I., Glantz G., Montroy R. The use of the carbon dioxide laser beam in the surgery of pressure sores. Int Surg. 1988 Jan-Mar;73(1):54–56. [PubMed] [Google Scholar]
- Faed E. M. Protein binding of drugs in plasma, interstitial fluid and tissues: effect on pharmacokinetics. Eur J Clin Pharmacol. 1981;21(1):77–81. doi: 10.1007/BF00609592. [DOI] [PubMed] [Google Scholar]
- Frankel H. L., Hancock D. O., Hyslop G., Melzak J., Michaelis L. S., Ungar G. H., Vernon J. D., Walsh J. J. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969 Nov;7(3):179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- Frisbie J. H., Kache A. Increasing survival and changing causes of death in myelopathy patients. J Am Paraplegia Soc. 1983 Jul;6(3):51–56. [PubMed] [Google Scholar]
- Galpin J. E., Chow A. W., Bayer A. S., Guze L. B. Sepsis associated with decubitus ulcers. Am J Med. 1976 Sep;61(3):346–350. doi: 10.1016/0002-9343(76)90371-5. [DOI] [PubMed] [Google Scholar]
- Gibaldi M., Levy G., McNamara P. J. Effect of plasma protein and tissue binding on the biologic half-life of drugs. Clin Pharmacol Ther. 1978 Jul;24(1):1–4. doi: 10.1002/cpt19782411. [DOI] [PubMed] [Google Scholar]
- Gonda I., Harpur E. S. Accumulation in the peripheral compartment of a linear two-compartment open model. J Pharmacokinet Biopharm. 1980 Feb;8(1):99–104. doi: 10.1007/BF01059451. [DOI] [PubMed] [Google Scholar]
- Greenway R. M., Houser H. B., Lindan O., Weir D. R. Long-term changes in gross body composition of paraplegic and quadriplegic patients. Paraplegia. 1970 Feb;7(4):301–318. doi: 10.1038/sc.1969.46. [DOI] [PubMed] [Google Scholar]
- Hoffstedt B., Walder M., Forsgren A. Comparison of skin blisters and implanted cotton threads for the evaluation of antibiotic tissue concentrations. Eur J Clin Microbiol. 1982 Feb;1(1):33–37. doi: 10.1007/BF02014138. [DOI] [PubMed] [Google Scholar]
- Kaplan J. M., McCracken G. H., Jr, Snyder E. Influence of methodology upon apparent concentrations of antibiotics in tissue. Antimicrob Agents Chemother. 1973 Feb;3(2):143–146. doi: 10.1128/aac.3.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekki M., Julkunen R. J., Pohjanpalo H. Pharmacokinetics of sulfaethidole in the rat: nonlinear multicompartment solution. J Pharmacokinet Biopharm. 1982 Feb;10(1):27–51. doi: 10.1007/BF01059182. [DOI] [PubMed] [Google Scholar]
- Kirby W. M., Clarke J. T., Libke R. D., Regamey C. Clinical pharmacology of amikacin and kanamycin. J Infect Dis. 1976 Nov;134(Suppl):S312–S315. doi: 10.1093/infdis/135.supplement_2.s312. [DOI] [PubMed] [Google Scholar]
- Mattie H., Hoogeterp J. J., Hermans J. The relation between plasma and tissue concentrations of antibiotics. Description of a method. J Pharmacokinet Biopharm. 1987 Apr;15(2):191–202. doi: 10.1007/BF01062343. [DOI] [PubMed] [Google Scholar]
- McCormack J. P., Schentag J. J. Potential impact of quantitative susceptibility tests on the design of aminoglycoside dosing regimens. Drug Intell Clin Pharm. 1987 Feb;21(2):187–192. [PubMed] [Google Scholar]
- Nuhlicek D. N., Spurr G. B., Barboriak J. J., Rooney C. B., el Ghatit A. Z., Bongard R. D. Body composition of patients with spinal cord injury. Eur J Clin Nutr. 1988 Sep;42(9):765–773. [PubMed] [Google Scholar]
- Peterson L. R., Gerding D. N., Moody J. A., Fasching C. E. Comparison of azlocillin, ceftizoxime, cefoxitin, and amikacin alone and in combination against Pseudomonas aeruginosa in a neutropenic-site rabbit model. Antimicrob Agents Chemother. 1984 May;25(5):545–552. doi: 10.1128/aac.25.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantin L. O., Ahlinder S., Norberg R., Birke G. The distribution of proteins between intra- and extravascular spaces in health and disease. Acta Med Scand. 1971 Apr;189(4):309–314. doi: 10.1111/j.0954-6820.1971.tb04381.x. [DOI] [PubMed] [Google Scholar]
- Plaue R., Bethke R. O., Fabricius K., Müller O. Kritische Untersuchungen zur Methodik von Antibiotikaspiegelbestimmungen in menschlichen Geweben. Arzneimittelforschung. 1980;30(1):1–5. [PubMed] [Google Scholar]
- Richardson R. R., Meyer P. R., Jr Prevalence and incidence of pressure sores in acute spinal cord injuries. Paraplegia. 1981;19(4):235–247. doi: 10.1038/sc.1981.47. [DOI] [PubMed] [Google Scholar]
- Rocci M. L., Jr, Jusko W. J. LAGRAN program for area and moments in pharmacokinetic analysis. Comput Programs Biomed. 1983 Jun;16(3):203–216. doi: 10.1016/0010-468x(83)90082-x. [DOI] [PubMed] [Google Scholar]
- Ryan D. M. Implanted cotton threads; a novel method for measuring concentrations of antibiotics in tissue fluid. J Antimicrob Chemother. 1979 Nov;5(6):735–737. doi: 10.1093/jac/5.6.735. [DOI] [PubMed] [Google Scholar]
- Schentag J. J., Jusko W. J., Plaut M. E., Cumbo T. J., Vance J. W., Abrutyn E. Tissue persistence of gentamicin in man. JAMA. 1977 Jul 25;238(4):327–329. [PubMed] [Google Scholar]
- Segal J. L., Brunnemann S. R., Gordon S. K., Eltorai I. M. Amikacin pharmacokinetics in patients with spinal cord injury. Pharmacotherapy. 1988;8(2):79–81. doi: 10.1002/j.1875-9114.1988.tb03539.x. [DOI] [PubMed] [Google Scholar]
- Segal J. L., Brunnemann S. R., Gray D. R. Gentamicin bioavailability and single-dose pharmacokinetics in spinal cord injury. Drug Intell Clin Pharm. 1988 Jun;22(6):461–465. doi: 10.1177/106002808802200603. [DOI] [PubMed] [Google Scholar]
- Stolley P. D., Strom B. L. Sample size calculations for clinical pharmacology studies. Clin Pharmacol Ther. 1986 May;39(5):489–490. doi: 10.1038/clpt.1986.85. [DOI] [PubMed] [Google Scholar]
- Tan J. S., Salstrom S. J., Signs S. A., Hoffman H. E., File T. M. Pharmacokinetics of intravenous cefmetazole with emphasis on comparison between predicted theoretical levels in tissue and actual skin window fluid levels. Antimicrob Agents Chemother. 1989 Jun;33(6):924–927. doi: 10.1128/aac.33.6.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. On pharmacokinetics in target tissues. Biopharm Drug Dispos. 1985 Jan-Mar;6(1):57–66. doi: 10.1002/bdd.2510060108. [DOI] [PubMed] [Google Scholar]
- Wise R., Andrews J. M. UK-18892, a new aminoglycoside: an in vitro study. Antimicrob Agents Chemother. 1978 Aug;14(2):228–233. doi: 10.1128/aac.14.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. Protein binding of beta-lactams: the effects on activity and pharmacology particularly tissue penetration. II. Studies in man. J Antimicrob Chemother. 1983 Aug;12(2):105–118. doi: 10.1093/jac/12.2.105. [DOI] [PubMed] [Google Scholar]
- Yamaoka K., Nakagawa T., Uno T. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978 Apr;6(2):165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]



