Abstract
Background—Interstitial cells of Cajal (ICCs) express the tyrosine kinase receptor c-kit, which is required for their development and spontaneous pacemaker activity in the bowel. From murine models it has been proposed that ICCs do not develop until after birth, but more recent findings indicate that c-kit is expressed early in the embryonic period. The temporal development of ICCs in the human gut remains unknown. Aim—To investigate ICCs in the human fetal small bowel using c-kit immunohistochemistry. Subjects—Small bowel specimens were obtained at post mortem examination of 16 fetuses and nine neonates, eight of whom were premature, born at gestational ages of 13 to 41 weeks, without gastrointestinal disorders. Methods—Immunohistochemical analysis was performed on material fixed in formalin and embedded in paraffin. The specimens were exposed to antibodies raised against c-kit (an ICC marker) and neurone specific enolase (a general neuronal marker). The ABC complex method was used to visualise binding of antibodies to the corresponding antigens. Results—c-kit immunoreactive cells were visualised from 13 weeks of gestation. The immunoreactivity was mainly localised in association with the myenteric plexus. From about 17-18 weeks of gestation, the ICCs formed a layer along the myenteric plexus, whereas this layer appeared to be disrupted at 13-16 weeks of gestation. Conclusions—ICCs are c-kit immunoreactive at least from a gestational age of 13 weeks in the human fetal small intestine. From 17-18 weeks of gestation until birth, they form a continuous layer around the myenteric ganglia.
Keywords: interstitial cells of Cajal; c-kit; myenteric plexus; human; fetal; development
Full Text
The Full Text of this article is available as a PDF (229.9 KB).
Figure 1 .
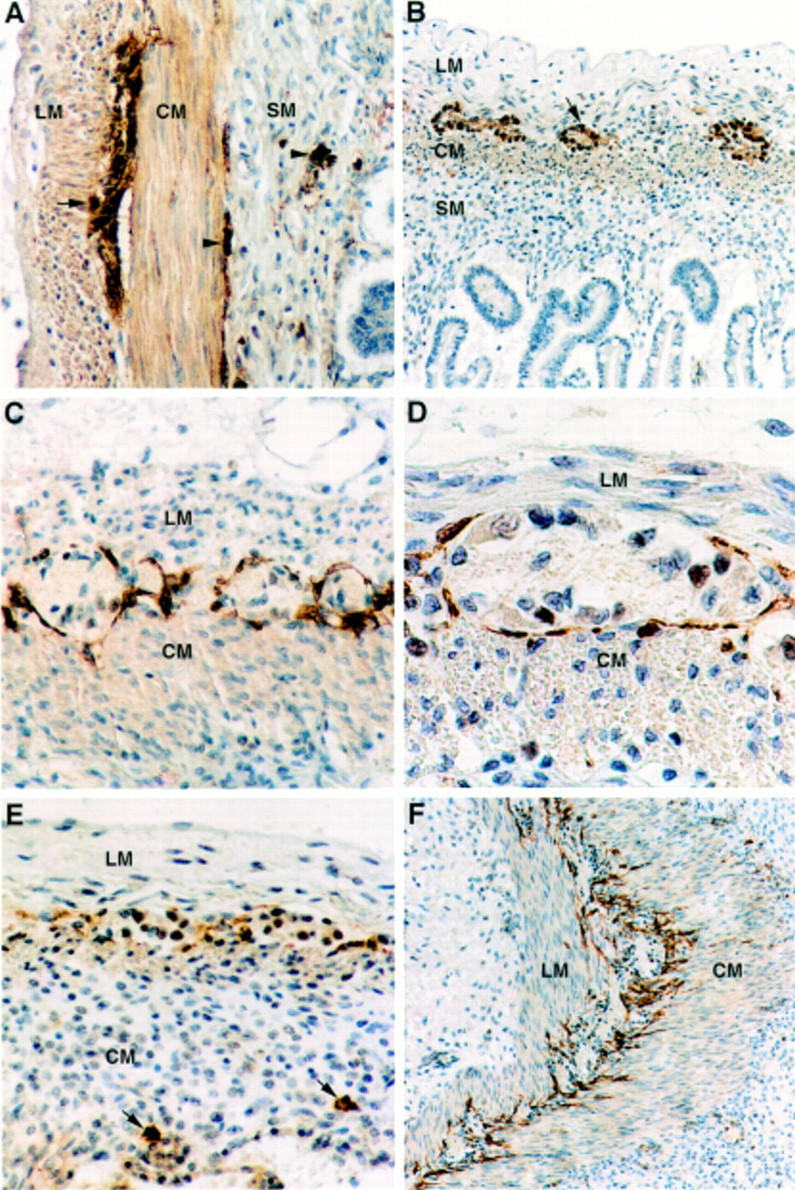
(A) NSE immunohistochemistry showing immunoreactive ganglion cells in the myenteric plexus (arrow) and in the inner and outer submucous plexuses (arrowheads) of the small bowel in an infant born at 26 weeks of gestation who died at the age of 17 days. Nerve fibres associated with the ganglia and in the circular muscle layer are also stained. Original magnification × 66. (B) NSE immunohistochemistry of the small bowel at 13 weeks of gestation displays the myenteric plexus (arrow), whereas no immunoreactive ganglion cells were present in the submucosa. Original magnification × 40. (C) From 17-18 weeks of gestation, the ICCs form a continuous layer along the myenteric plexus of the small bowel. This is illustrated in a case at 20 weeks of gestation using c-kit immunohistochemistry. Original magnification × 80. (D) c-kit immunoreactive cells are elongated in shape and have an ovoid nucleus as in this case at 20 weeks of gestation. The cell processes do not seem to penetrate into the ganglia. Original magnification × 200. (E) From 13 to 16 weeks of gestation, the layer of c-kit immunoreactive ICCs associated with the myenteric plexus of the small bowel appears to be disrupted. In the submucosa, isolated round c-kit immunoreactive cells, interpreted as mast cells, are seen (arrows). In this case the submucous plexus had not yet developed at 13 weeks of gestation, as shown in (B). Original magnification × 80. (F) From 17 weeks of gestation, the processes of the ICCs are often seen to penetrate into the circular muscle layer, whereas isolated ICCs in this layer are only rarely observed. Original magnification × 50. Abbreviations: LM, longitudinal muscle layer; CM, circular muscle layer; SM, submucosa.
Figure 2 .
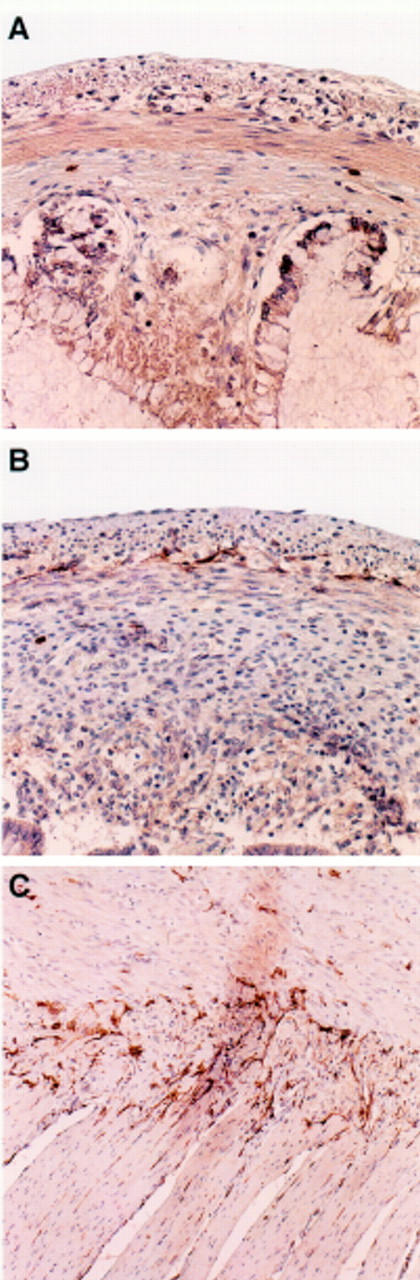
(A) At 13 weeks of gestation no c-kit immunoreactivity can be seen in association with the myenteric plexus of the rectum. Original magnification × 50. (B) A layer of c-kit immunoreactive ICCs is seen around the myenteric plexus of the rectum at 19 weeks of gestation. Original magnification × 50. (C) In the adult small bowel, c-kit immunoreactive ICCs surround the myenteric ganglia. ICCs are also frequently found in the circular muscle layer. Original magnification × 25.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berseth C. L. Gestational evolution of small intestine motility in preterm and term infants. J Pediatr. 1989 Oct;115(4):646–651. doi: 10.1016/s0022-3476(89)80302-6. [DOI] [PubMed] [Google Scholar]
- Berseth C. L. Neonatal small intestinal motility: motor responses to feeding in term and preterm infants. J Pediatr. 1990 Nov;117(5):777–782. doi: 10.1016/s0022-3476(05)83343-8. [DOI] [PubMed] [Google Scholar]
- Bisset W. M., Watt J. B., Rivers R. P., Milla P. J. Ontogeny of fasting small intestinal motor activity in the human infant. Gut. 1988 Apr;29(4):483–488. doi: 10.1136/gut.29.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns A. J., Herbert T. M., Ward S. M., Sanders K. M. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit immunohistochemistry. Cell Tissue Res. 1997 Oct;290(1):11–20. doi: 10.1007/s004410050902. [DOI] [PubMed] [Google Scholar]
- Der-Silaphet T., Malysz J., Hagel S., Larry Arsenault A., Huizinga J. D. Interstitial cells of cajal direct normal propulsive contractile activity in the mouse small intestine. Gastroenterology. 1998 Apr;114(4):724–736. doi: 10.1016/s0016-5085(98)70586-4. [DOI] [PubMed] [Google Scholar]
- Durdle N. G., Kingma Y. J., Bowes K. L., Chambers M. M. Origin of slow waves in the canine colon. Gastroenterology. 1983 Feb;84(2):375–382. [PubMed] [Google Scholar]
- Faussone-Pellegrini M. S. Cytodifferentiation of the interstitial cells of Cajal related to the myenteric plexus of mouse intestinal muscle coat. An E.M. study from foetal to adult life. Anat Embryol (Berl) 1985;171(2):163–169. doi: 10.1007/BF00341410. [DOI] [PubMed] [Google Scholar]
- Fekete E., Benedeczky I., Timmermans J. P., Resch B. A., Scheuermann D. W. Sequential pattern of nerve-muscle contacts in the small intestine of developing human fetus. An ultrastructural and immunohistochemical study. Histol Histopathol. 1996 Oct;11(4):845–850. [PubMed] [Google Scholar]
- Flanagan J. G., Leder P. The kit ligand: a cell surface molecule altered in steel mutant fibroblasts. Cell. 1990 Oct 5;63(1):185–194. doi: 10.1016/0092-8674(90)90299-t. [DOI] [PubMed] [Google Scholar]
- Horie K., Fujita J., Takakura K., Kanzaki H., Suginami H., Iwai M., Nakayama H., Mori T. The expression of c-kit protein in human adult and fetal tissues. Hum Reprod. 1993 Nov;8(11):1955–1962. doi: 10.1093/oxfordjournals.humrep.a137967. [DOI] [PubMed] [Google Scholar]
- Huizinga J. D., Thuneberg L., Klüppel M., Malysz J., Mikkelsen H. B., Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995 Jan 26;373(6512):347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Isozaki K., Hirota S., Miyagawa J., Taniguchi M., Shinomura Y., Matsuzawa Y. Deficiency of c-kit+ cells in patients with a myopathic form of chronic idiopathic intestinal pseudo-obstruction. Am J Gastroenterol. 1997 Feb;92(2):332–334. [PubMed] [Google Scholar]
- Knudsen L. M., Pallesen G. The preservation and loss of various non-haematopoietic antigens in human post-mortem tissues as demonstrated by monoclonal antibody immunohistological staining. Histopathology. 1986 Oct;10(10):1007–1014. doi: 10.1111/j.1365-2559.1986.tb02537.x. [DOI] [PubMed] [Google Scholar]
- Lecoin L., Gabella G., Le Douarin N. Origin of the c-kit-positive interstitial cells in the avian bowel. Development. 1996 Mar;122(3):725–733. doi: 10.1242/dev.122.3.725. [DOI] [PubMed] [Google Scholar]
- Liu L. W., Huizinga J. D. Electrical coupling of circular muscle to longitudinal muscle and interstitial cells of Cajal in canine colon. J Physiol. 1993 Oct;470:445–461. doi: 10.1113/jphysiol.1993.sp019868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. W., Thuneberg L., Huizinga J. D. Selective lesioning of interstitial cells of Cajal by methylene blue and light leads to loss of slow waves. Am J Physiol. 1994 Mar;266(3 Pt 1):G485–G496. doi: 10.1152/ajpgi.1994.266.3.G485. [DOI] [PubMed] [Google Scholar]
- MCLAIN C. R., Jr AMNIOGRAPHY STUDIES OF THE GASTROINTESTINAL MOTILITY OF THE HUMAN FETUS. Am J Obstet Gynecol. 1963 Aug 15;86:1079–1087. doi: 10.1016/s0002-9378(16)35300-5. [DOI] [PubMed] [Google Scholar]
- Maeda H., Yamagata A., Nishikawa S., Yoshinaga K., Kobayashi S., Nishi K., Nishikawa S. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992 Oct;116(2):369–375. doi: 10.1242/dev.116.2.369. [DOI] [PubMed] [Google Scholar]
- Matsuda R., Takahashi T., Nakamura S., Sekido Y., Nishida K., Seto M., Seito T., Sugiura T., Ariyoshi Y., Takahashi T. Expression of the c-kit protein in human solid tumors and in corresponding fetal and adult normal tissues. Am J Pathol. 1993 Jan;142(1):339–346. [PMC free article] [PubMed] [Google Scholar]
- Prosser C. L., Holzwarth M. A., Barr L. Immunocytochemistry of the interstitial cells of Cajal in the rat intestine. J Auton Nerv Syst. 1989 Jun;27(1):17–25. doi: 10.1016/0165-1838(89)90124-0. [DOI] [PubMed] [Google Scholar]
- Publicover N. G., Hammond E. M., Sanders K. M. Amplification of nitric oxide signaling by interstitial cells isolated from canine colon. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2087–2091. doi: 10.1073/pnas.90.5.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumessen J. J., Mikkelsen H. B., Qvortrup K., Thuneberg L. Ultrastructure of interstitial cells of Cajal in circular muscle of human small intestine. Gastroenterology. 1993 Feb;104(2):343–350. doi: 10.1016/0016-5085(93)90400-7. [DOI] [PubMed] [Google Scholar]
- Rumessen J. J., Mikkelsen H. B., Thuneberg L. Ultrastructure of interstitial cells of Cajal associated with deep muscular plexus of human small intestine. Gastroenterology. 1992 Jan;102(1):56–68. doi: 10.1016/0016-5085(92)91784-2. [DOI] [PubMed] [Google Scholar]
- Rumessen J. J., Thuneberg L. Interstitial cells of Cajal in human small intestine. Ultrastructural identification and organization between the main smooth muscle layers. Gastroenterology. 1991 May;100(5 Pt 1):1417–1431. [PubMed] [Google Scholar]
- Thuneberg L. Interstitial cells of Cajal: intestinal pacemaker cells? Adv Anat Embryol Cell Biol. 1982;71:1–130. [PubMed] [Google Scholar]
- Torihashi S., Ward S. M., Sanders K. M. Development of c-Kit-positive cells and the onset of electrical rhythmicity in murine small intestine. Gastroenterology. 1997 Jan;112(1):144–155. doi: 10.1016/s0016-5085(97)70229-4. [DOI] [PubMed] [Google Scholar]
- Vanderwinden J. M., Liu H., De Laet M. H., Vanderhaeghen J. J. Study of the interstitial cells of Cajal in infantile hypertrophic pyloric stenosis. Gastroenterology. 1996 Aug;111(2):279–288. doi: 10.1053/gast.1996.v111.pm8690192. [DOI] [PubMed] [Google Scholar]
- Vanderwinden J. M., Rumessen J. J., Liu H., Descamps D., De Laet M. H., Vanderhaeghen J. J. Interstitial cells of Cajal in human colon and in Hirschsprung's disease. Gastroenterology. 1996 Oct;111(4):901–910. doi: 10.1016/s0016-5085(96)70057-4. [DOI] [PubMed] [Google Scholar]
- Ward S. M., Burke E. P., Sanders K. M. Use of rhodamine 123 to label and lesion interstitial cells of Cajal in canine colonic circular muscle. Anat Embryol (Berl) 1990;182(3):215–224. doi: 10.1007/BF00185515. [DOI] [PubMed] [Google Scholar]
- Wester T., O'Briain S., Puri P. NADPH diaphorase-containing nerve fibers and neurons in the myenteric plexus are resistant to postmortem changes: studies in Hirschsprung's disease and normal autopsy material. Arch Pathol Lab Med. 1998 May;122(5):461–466. [PubMed] [Google Scholar]
- Williams D. E., Eisenman J., Baird A., Rauch C., Van Ness K., March C. J., Park L. S., Martin U., Mochizuki D. Y., Boswell H. S. Identification of a ligand for the c-kit proto-oncogene. Cell. 1990 Oct 5;63(1):167–174. doi: 10.1016/0092-8674(90)90297-r. [DOI] [PubMed] [Google Scholar]
- Xue C., Pollock J., Schmidt H. H., Ward S. M., Sanders K. M. Expression of nitric oxide synthase immunoreactivity by interstitial cells of the canine proximal colon. J Auton Nerv Syst. 1994 Sep;49(1):1–14. doi: 10.1016/0165-1838(94)90015-9. [DOI] [PubMed] [Google Scholar]
- Yamataka A., Kato Y., Tibboel D., Murata Y., Sueyoshi N., Fujimoto T., Nishiye H., Miyano T. A lack of intestinal pacemaker (c-kit) in aganglionic bowel of patients with Hirschsprung's disease. J Pediatr Surg. 1995 Mar;30(3):441–444. doi: 10.1016/0022-3468(95)90051-9. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Kuang W. J., Yang-Feng T., Coussens L., Munemitsu S., Dull T. J., Chen E., Schlessinger J., Francke U., Ullrich A. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987 Nov;6(11):3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsebo K. M., Wypych J., McNiece I. K., Lu H. S., Smith K. A., Karkare S. B., Sachdev R. K., Yuschenkoff V. N., Birkett N. C., Williams L. R. Identification, purification, and biological characterization of hematopoietic stem cell factor from buffalo rat liver--conditioned medium. Cell. 1990 Oct 5;63(1):195–201. doi: 10.1016/0092-8674(90)90300-4. [DOI] [PubMed] [Google Scholar]


