Abstract
Background—Cholera toxin causes small intestinal hypersecretion by inducing a coordinated response from enterocytes, enterochromaffin cells, enteric neurones, and the vascular supply. Nitric oxide has been implicated in the function of these separate components. Aims—To explore the role of nitric oxide in the totality of cholera toxin induced secretion in vivo. Methods—One group of adult male Wistar rats was treated with the nitric oxide synthase inhibitors NG-nitro-L-arginine methyl ester (L-NAME; subcutaneously or intraluminally), NG-methyl-L-arginine (L-NMA), or 7-nitroindazole. A second group of rats was treated with L-arginine (intraperitoneally or intraluminally) or D-arginine. The small intestine was isolated between two cannulae and instilled with 75 µg cholera toxin or saline for two hours. Small intestinal perfusion of a plasma electrolyte solution containing [14C]-PEG was undertaken to determine net water and electrolyte movement. After the experiment macroscopic and microscopic intestinal appearances were noted and jejunal 5-hydroxytryptamine concentrations were determined. Results—Both L-arginine and L-NAME induced secretion in the basal state, but only when administered intraluminally. Systemically applied L-NAME caused a dose dependent reduction in cholera toxin induced secretion. This was paralleled by L-NMA but not by 7-nitroindazole or by intraluminally applied L-NAME. Systemically applied L-NAME caused notable cyanosis of the intestine, consistent with mesenteric ischaemia, but no microscopic abnormalities. Systemically applied L-arginine but not D-arginine also reduced cholera toxin induced secretion and inhibited 5-hydroxytryptamine release. Conclusion—Nitric oxide has a duality of roles in cholera toxin induced secretion, acting both as an absorbagogue and a secretagogue. Its mechanisms of action include the maintenance of mucosal perfusion and enterochromaffin cell stabilisation.
Keywords: cholera toxin; nitric oxide; small intestinal transport; 5-hydroxytryptamine; L-arginine; nitric oxide synthase inhibitors
Full Text
The Full Text of this article is available as a PDF (131.5 KB).
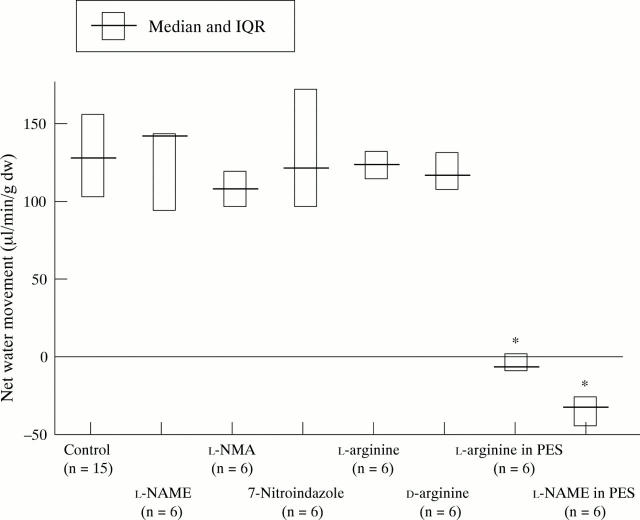
Figure 1 .
Influence of systemic L-NAME (60 mg/kg), L-NMA (50 mg/kg), 7-nitroindazole (100 mg/kg), L-arginine (1 g/kg), and D-arginine (1 g/kg) and intraluminal L-NAME and L-arginine (20 mmol/l added to PES) on basal net small intestinal water movement. *p<0.0001 compared with control.
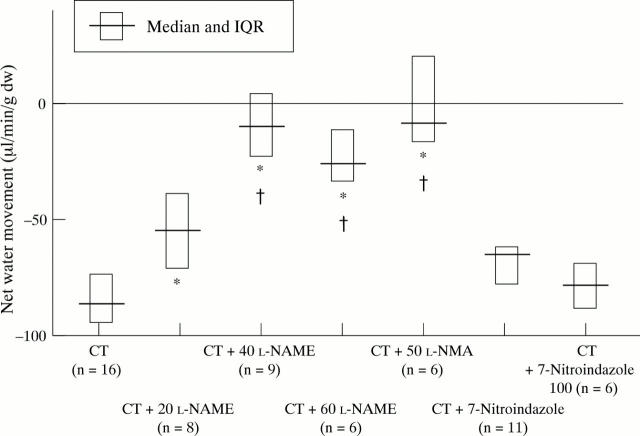
Figure 2 .
Influence of L-NAME (20, 40, and 60 mg/kg), L-NMA (50 mg/kg), and 7-nitroindazole (50 and 100 mg/kg) on cholera toxin (CT) induced net small intestinal water movement (µl/min/g dry intestinal weight). *p<0.02 compared with CT; †p<0.02 compared with CT+L-NAME 20 mg/kg; NS, CT versus CT+7-nitroindazole.
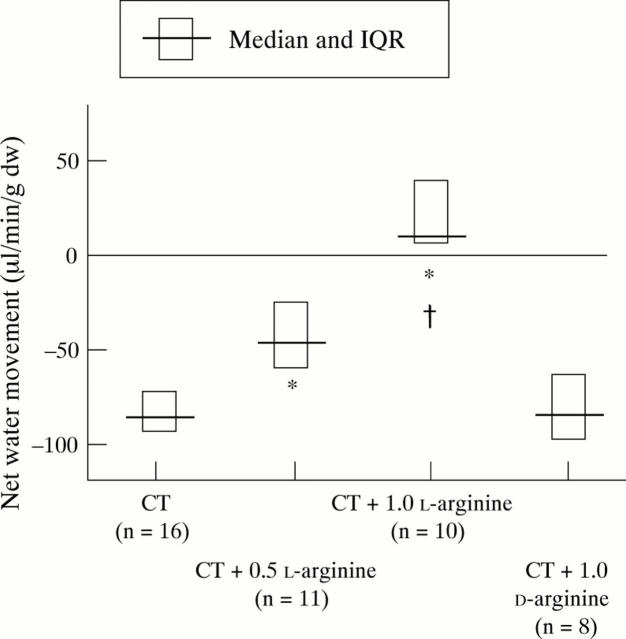
Figure 3 .
Influence of L-arginine 0.5 and 1.0 g/kg and D-arginine 1.0 g/kg on cholera toxin (CT) induced net small intestinal water movement.*p<0.002 compared with control; †p<0.0003 compared with CT+L-arginine 0.5 g/kg; NS, CT versus CT+D-arginine.
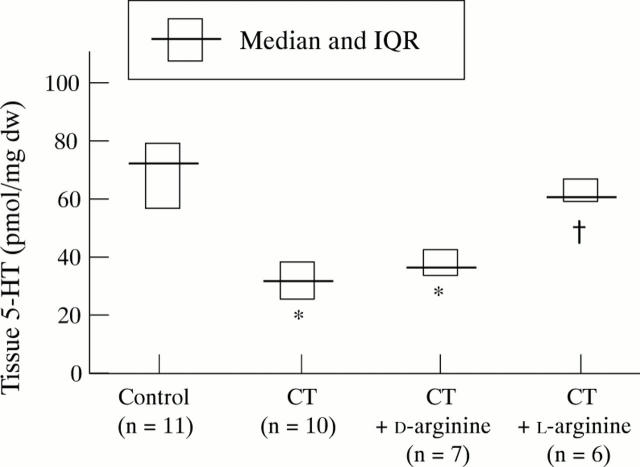
Figure 4 .
Effect of pretreatment with L-arginine or D-arginine (1 g/kg) on cholera toxin (CT) induced decrease in small intestinal 5-HT concentrations. *p<0.0001 compared with control; †p<0.0002 compared with CT and CT+D-arginine; NS, control versus CT+L-arginine.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes J. M., Barnes N. M., Costall B., Naylor R. J., Tattersall F. D. Reserpine, para-chlorophenylalanine and fenfluramine antagonise cisplatin-induced emesis in the ferret. Neuropharmacology. 1988 Aug;27(8):783–790. doi: 10.1016/0028-3908(88)90092-5. [DOI] [PubMed] [Google Scholar]
- Barry M. K., Aloisi J. D., Pickering S. P., Yeo C. J. Nitric oxide modulates water and electrolyte transport in the ileum. Ann Surg. 1994 Apr;219(4):382–388. doi: 10.1097/00000658-199404000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearcroft C. P., Farthing M. J., Perrett D. Determination of 5-hydroxytryptamine, 5-hydroxyindoleacetic acid and tryptophan in plasma and urine by HPLC with fluorimetric detection. Biomed Chromatogr. 1995 Jan-Feb;9(1):23–27. doi: 10.1002/bmc.1130090105. [DOI] [PubMed] [Google Scholar]
- Bearcroft C. P., Perrett D., Farthing M. J. 5-hydroxytryptamine release into human jejunum by cholera toxin. Gut. 1996 Oct;39(4):528–531. doi: 10.1136/gut.39.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beubler E., Horina G. 5-HT2 and 5-HT3 receptor subtypes mediate cholera toxin-induced intestinal fluid secretion in the rat. Gastroenterology. 1990 Jul;99(1):83–89. doi: 10.1016/0016-5085(90)91233-v. [DOI] [PubMed] [Google Scholar]
- Beubler E., Schirgi-Degen A. Nitric oxide counteracts 5-hydroxytryptamine- and cholera toxin-induced fluid secretion and enhances the effect of oral rehydration solution. Eur J Pharmacol. 1997 May 20;326(2-3):223–228. doi: 10.1016/s0014-2999(97)85417-9. [DOI] [PubMed] [Google Scholar]
- Bohlen H. G., Lash J. M. Intestinal absorption of sodium and nitric oxide-dependent vasodilation interact to dominate resting vascular resistance. Circ Res. 1996 Feb;78(2):231–237. doi: 10.1161/01.res.78.2.231. [DOI] [PubMed] [Google Scholar]
- Brittain T., Blackmore R., Greenwood C., Thomson A. J. Bacterial nitrite-reducing enzymes. Eur J Biochem. 1992 Nov 1;209(3):793–802. doi: 10.1111/j.1432-1033.1992.tb17350.x. [DOI] [PubMed] [Google Scholar]
- Caplan M. S., Hedlund E., Hill N., MacKendrick W. The role of endogenous nitric oxide and platelet-activating factor in hypoxia-induced intestinal injury in rats. Gastroenterology. 1994 Feb;106(2):346–352. doi: 10.1016/0016-5085(94)90591-6. [DOI] [PubMed] [Google Scholar]
- Cassuto J., Jodal M., Tuttle R., Lundgren O. On the role of intramural nerves in the pathogenesis of cholera toxin-induced intestinal secretion. Scand J Gastroenterol. 1981 Apr;16(3):377–384. doi: 10.3109/00365528109181984. [DOI] [PubMed] [Google Scholar]
- Drexler H., Zeiher A. M., Meinzer K., Just H. Correction of endothelial dysfunction in coronary microcirculation of hypercholesterolaemic patients by L-arginine. Lancet. 1991 Dec 21;338(8782-8783):1546–1550. doi: 10.1016/0140-6736(91)92372-9. [DOI] [PubMed] [Google Scholar]
- Elliott E. J., Watson A. J., Walker-Smith J. A., Farthing M. J. Search for the ideal oral rehydration solution: studies in a model of secretory diarrhoea. Gut. 1991 Nov;32(11):1314–1320. doi: 10.1136/gut.32.11.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M. Modes of action of enterotoxins from Vibrio cholerae and EScherichia coli. Rev Infect Dis. 1979 Nov-Dec;1(6):918–926. doi: 10.1093/clinids/1.6.918. [DOI] [PubMed] [Google Scholar]
- Field M., Rao M. C., Chang E. B. Intestinal electrolyte transport and diarrheal disease (1). N Engl J Med. 1989 Sep 21;321(12):800–806. doi: 10.1056/NEJM198909213211206. [DOI] [PubMed] [Google Scholar]
- Field M., Rao M. C., Chang E. B. Intestinal electrolyte transport and diarrheal disease (2) N Engl J Med. 1989 Sep 28;321(13):879–883. doi: 10.1056/NEJM198909283211307. [DOI] [PubMed] [Google Scholar]
- Geiger J., Nolte C., Butt E., Sage S. O., Walter U. Role of cGMP and cGMP-dependent protein kinase in nitrovasodilator inhibition of agonist-evoked calcium elevation in human platelets. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1031–1035. doi: 10.1073/pnas.89.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. B., Skadhauge E. Ketanserin and granisetron reduce cholera toxin-induced hypersecretion in pig jejunum. Scand J Gastroenterol. 1994 Oct;29(10):908–915. doi: 10.3109/00365529409094862. [DOI] [PubMed] [Google Scholar]
- Hecker M., Sessa W. C., Harris H. J., Anggård E. E., Vane J. R. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: cultured endothelial cells recycle L-citrulline to L-arginine. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8612–8616. doi: 10.1073/pnas.87.21.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty J. E., Fairclough P. D., Clark M. L., Dawson A. M. Jejunal water and electrolyte secretion induced by L-arginine in man. Gut. 1981 Feb;22(2):108–113. doi: 10.1136/gut.22.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellier M. D., Thirumalai C., Holdsworth C. D. The effect of amino acids and dipeptides on sodium and water absorption in man. Gut. 1973 Jan;14(1):41–45. doi: 10.1136/gut.14.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry Y., Lepoivre M., Drapier J. C., Ducrocq C., Boucher J. L., Guissani A. EPR characterization of molecular targets for NO in mammalian cells and organelles. FASEB J. 1993 Sep;7(12):1124–1134. doi: 10.1096/fasebj.7.12.8397130. [DOI] [PubMed] [Google Scholar]
- Izzo A. A., Gaginella T. S., Mascolo N., Capasso F. Nitric oxide as a mediator of the laxative action of magnesium sulphate. Br J Pharmacol. 1994 Sep;113(1):228–232. doi: 10.1111/j.1476-5381.1994.tb16198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodal M., Lundgren O. Countercurrent mechanisms in the mammalian gastrointestinal tract. Gastroenterology. 1986 Jul;91(1):225–241. doi: 10.1016/0016-5085(86)90463-4. [DOI] [PubMed] [Google Scholar]
- Jodal M. Neuronal influence on intestinal transport. J Intern Med Suppl. 1990;732:125–132. doi: 10.1111/j.1365-2796.1990.tb01484.x. [DOI] [PubMed] [Google Scholar]
- Kanwar S., Wallace J. L., Befus D., Kubes P. Nitric oxide synthesis inhibition increases epithelial permeability via mast cells. Am J Physiol. 1994 Feb;266(2 Pt 1):G222–G229. doi: 10.1152/ajpgi.1994.266.2.G222. [DOI] [PubMed] [Google Scholar]
- King C. A., Van Heyningen W. E. Deactivation of cholera toxin by a sialidase-resistant monosialosylganglioside. J Infect Dis. 1973 Jun;127(6):639–647. doi: 10.1093/infdis/127.6.639. [DOI] [PubMed] [Google Scholar]
- Li Y. F., Weisbrodt N. W., Lodato R. F., Moody F. G. Nitric oxide is involved in muscle relaxation but not in changes in short-circuit current in rat ileum. Am J Physiol. 1994 Apr;266(4 Pt 1):G554–G559. doi: 10.1152/ajpgi.1994.266.4.G554. [DOI] [PubMed] [Google Scholar]
- Lowenstein C. J., Dinerman J. L., Snyder S. H. Nitric oxide: a physiologic messenger. Ann Intern Med. 1994 Feb 1;120(3):227–237. doi: 10.7326/0003-4819-120-3-199402010-00009. [DOI] [PubMed] [Google Scholar]
- Lundgren O., Svanvik J., Jivegård L. Enteric nervous system. I. Physiology and pathophysiology of the intestinal tract. Dig Dis Sci. 1989 Feb;34(2):264–283. doi: 10.1007/BF01536062. [DOI] [PubMed] [Google Scholar]
- M'Rabet-Touil H., Blachier F., Morel M. T., Darcy-Vrillon B., Duée P. H. Characterization and ontogenesis of nitric oxide synthase activity in pig enterocytes. FEBS Lett. 1993 Oct 4;331(3):243–247. doi: 10.1016/0014-5793(93)80345-u. [DOI] [PubMed] [Google Scholar]
- Maher M. M., Gontarek J. D., Jimenez R. E., Cahill P. A., Yeo C. J. Endogenous nitric oxide promotes ileal absorption. J Surg Res. 1995 Jun;58(6):687–692. doi: 10.1006/jsre.1995.1108. [DOI] [PubMed] [Google Scholar]
- Mailman D. Differential effects of lumenal L-arginine and NG-nitro L-arginine on blood flow and water fluxes in rat ileum. Br J Pharmacol. 1994 May;112(1):304–310. doi: 10.1111/j.1476-5381.1994.tb13069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascolo N., Izzo A. A., Barbato F., Capasso F. Inhibitors of nitric oxide synthetase prevent castor-oil-induced diarrhoea in the rat. Br J Pharmacol. 1993 Apr;108(4):861–864. doi: 10.1111/j.1476-5381.1993.tb13478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathan M. M., Chandy G., Mathan V. I. Ultrastructural changes in the upper small intestinal mucosa in patients with cholera. Gastroenterology. 1995 Aug;109(2):422–430. doi: 10.1016/0016-5085(95)90329-1. [DOI] [PubMed] [Google Scholar]
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993 Dec 30;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Mourad F. H., O'Donnell L. J., Andre E. A., Bearcroft C. P., Owen R. A., Clark M. L., Farthing M. J. L-Arginine, nitric oxide, and intestinal secretion: studies in rat jejunum in vivo. Gut. 1996 Oct;39(4):539–544. doi: 10.1136/gut.39.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourad F. H., O'Donnell L. J., Dias J. A., Ogutu E., Andre E. A., Turvill J. L., Farthing M. J. Role of 5-hydroxytryptamine type 3 receptors in rat intestinal fluid and electrolyte secretion induced by cholera and Escherichia coli enterotoxins. Gut. 1995 Sep;37(3):340–345. doi: 10.1136/gut.37.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Ney P., Schröder H., Schrör K. Nitrovasodilator-induced inhibition of LTB4 release from human PMN may be mediated by cyclic GMP. Eicosanoids. 1990;3(4):243–245. [PubMed] [Google Scholar]
- Nichols K., Staines W., Krantis A. Nitric oxide synthase distribution in the rat intestine: a histochemical analysis. Gastroenterology. 1993 Dec;105(6):1651–1661. doi: 10.1016/0016-5085(93)91060-u. [DOI] [PubMed] [Google Scholar]
- Nilsson O., Cassuto J., Larsson P. A., Jodal M., Lidberg P., Ahlman H., Dahlström A., Lundgren O. 5-Hydroxytryptamine and cholera secretion: a histochemical and physiological study in cats. Gut. 1983 Jun;24(6):542–548. doi: 10.1136/gut.24.6.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaka M., Fujita T., Yanatori Y. On the possible role of intestinal hormones as the diarrhoeagenic messenger in cholera. Virchows Arch B Cell Pathol. 1975 Sep 11;18(4):287–296. doi: 10.1007/BF02889255. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Qiu B., Pothoulakis C., Castagliuolo I., Nikulasson Z., LaMont J. T. Nitric oxide inhibits rat intestinal secretion by Clostridium difficile toxin A but not Vibrio cholerae enterotoxin. Gastroenterology. 1996 Aug;111(2):409–418. doi: 10.1053/gast.1996.v111.pm8690206. [DOI] [PubMed] [Google Scholar]
- Rand M. J. Nitrergic transmission: nitric oxide as a mediator of non-adrenergic, non-cholinergic neuro-effector transmission. Clin Exp Pharmacol Physiol. 1992 Mar;19(3):147–169. doi: 10.1111/j.1440-1681.1992.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Rao R. K., Riviere P. J., Pascaud X., Junien J. L., Porreca F. Tonic regulation of mouse ileal ion transport by nitric oxide. J Pharmacol Exp Ther. 1994 May;269(2):626–631. [PubMed] [Google Scholar]
- Rolfe V., Levin R. J. Enterotoxin Escherichia coli STa activates a nitric oxide-dependent myenteric plexus secretory reflex in the rat ileum. J Physiol. 1994 Mar 15;475(3):531–537. doi: 10.1113/jphysiol.1994.sp020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolston D. D., Borodo M. M., Kelly M. J., Dawson A. M., Farthing M. J. Efficacy of oral rehydration solutions in a rat model of secretory diarrhea. J Pediatr Gastroenterol Nutr. 1987 Jul-Aug;6(4):624–630. doi: 10.1097/00005176-198707000-00023. [DOI] [PubMed] [Google Scholar]
- Salzman A. L. Nitric oxide in the gut. New Horiz. 1995 Feb;3(1):33–45. [PubMed] [Google Scholar]
- Schirgi-Degen A., Beubler E. Significance of nitric oxide in the stimulation of intestinal fluid absorption in the rat jejunum in vivo. Br J Pharmacol. 1995 Jan;114(1):13–18. doi: 10.1111/j.1476-5381.1995.tb14899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallance P., Collier J., Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989 Oct 28;2(8670):997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Limmroth V., Irikura K., Moskowitz M. A. The NOS inhibitor, 7-nitroindazole, decreases focal infarct volume but not the response to topical acetylcholine in pial vessels. J Cereb Blood Flow Metab. 1994 Nov;14(6):924–929. doi: 10.1038/jcbfm.1994.123. [DOI] [PubMed] [Google Scholar]