Abstract
Background—Extracellular matrix (ECM) degradation may play a role in villus atrophy in coeliac disease (CD). Aims—To compare the cellular expression of mRNA transcripts for the two major matrix degrading proteases, matrix metalloproteinase (MMP)-1 and MMP-3, their inhibitor, tissue inhibitor of metalloproteinases (TIMP)-1, and procollagen I in the intestinal mucosa of patients with untreated and treated CD and normal controls. Patients/Methods—Duodenal biopsy specimens from ten untreated CD patients, from six of these after a gluten free diet, and from ten control patients were hybridised with 35S-labelled RNA probes. The number of positive cells in the subepithelial region and lamina propria were counted microscopically. Results—The numbers of cells positive for MMP-1 (p<0.005), MMP-3 (p<0.01), and TIMP-1 (p<0.05) mRNA were higher in the subepithelial region of CD mucosa than in that from controls. In the lamina propria, only cells positive for MMP-1 mRNA were increased in CD patients compared with controls (p<0.01). MMP-1 and MMP-3 mRNA expression returned to normal in CD patients after treatment with a gluten free diet (p<0.05), while TIMP-1 mRNA expression remained elevated. The number of procollagen I mRNA expressing cells did not change. Expression of MMP-1 and MMP-3 mRNA was mainly localised to subepithelial fibroblasts and macrophages. Conclusions—The decreased ratio of collagen I and TIMP-1 mRNA expressing cells to MMP-1 and MMP-3 mRNA expressing cells in untreated CD suggests a shift towards ECM degradation. ECM degradation by activated subepithelial fibroblasts and macrophages may be an important mechanism driving mucosal transformation in CD.
Keywords: coeliac disease; extracellular matrix; villus atrophy; matrix metalloproteinases; TIMP-1; collagen I
Full Text
The Full Text of this article is available as a PDF (311.8 KB).
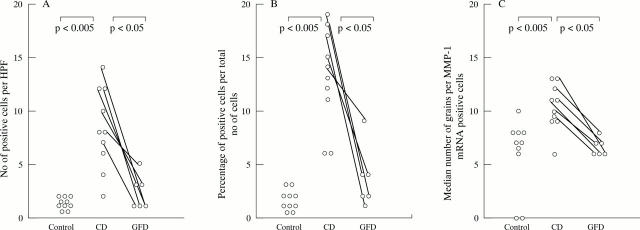
Figure 1 .
The number (A) and proportion (B) of cells expressing matrix metalloproteinase-1 (MMP-1) mRNA is significantly increased in patients with coeliac disease (CD) compared with the controls, in both the subepithelial layer (SEL) and the lamina propria (LP). A significant reduction in MMP-1 transcripts in the subepithelial cell layer was found in biopsy specimens taken from CD patients on a gluten free diet (GFD) in comparison with untreated patients. The number of positive cells in the subepithelial region and the lamina propria were counted per high power field at a 400-fold magnification. (C) MMP-1 mRNA expression per positive cell is also increased in untreated CD compared with controls and compared with after treatment with a gluten free diet. Results are expressed as number of positive cells per high power field (HPF) (A), percentage of positive cells per total number of cells (B), and number of grains per positive cell (C). Results are from 10 patients with CD, 10 controls, and six of the CD patients after treatment with a gluten free diet.
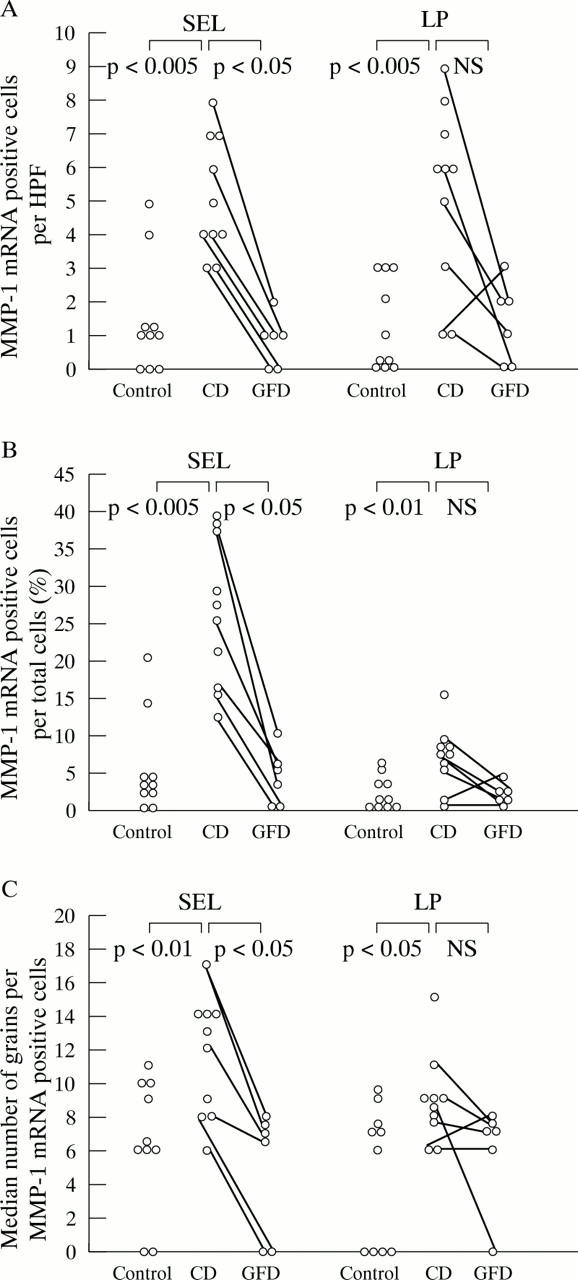
Figure 2 .
(A) and (B) Expression of matrix metalloproteinase-3 (MMP-3) mRNA is significantly increased in the subepithelial layer (number of positive cells per high power field (HPF) and percentage of total number of cells that were positive) and in the lamina propria in patients with coeliac disease (CD) compared with controls (positive cells per high power field). The reduction in MMP-3 transcripts in CD patients after a gluten free diet (GFD) is only significant in the lamina propria (B). (C) In biopsy specimens from untreated CD patients compared with controls and after treatment with a gluten free diet, the MMP-3 mRNA expression per positive cell is increased in the subepithelial layer, but not in the lamina propria. For further explanations, see fig 1.
Figure 3 .
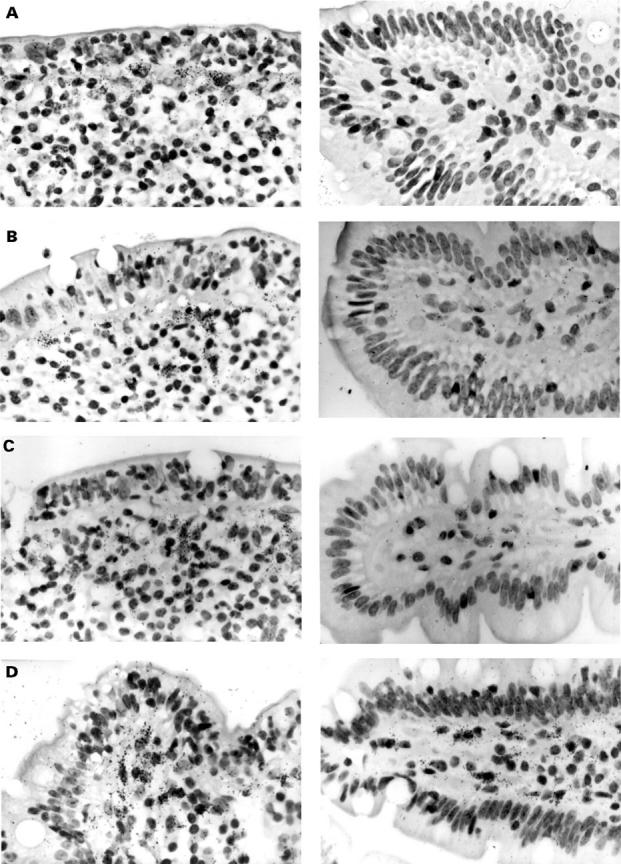
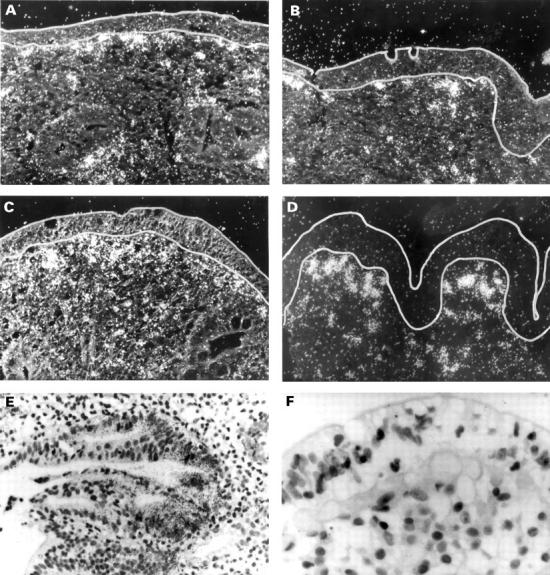
Localisation of matrix metalloproteinase (MMP)-1 (A), MMP-3 (B), tissue inhibitor of metalloproteinases (TIMP)-1 (C), and collagen I (D) mRNA in the duodenal mucosa in coeliac disease (CD) (left) and uninflamed control tissue (right) by in situ hybridisation. Black (white in dark field photographs in CD) grains indicate hybridisation of RNA antisense probes to mRNA in the tissue. In CD, expression of MMP-1 and MMP-3 mRNA was found almost exclusively in the subepithelial cell layer and the adjacent region of the lamina propria. MMP-1 and MMP-3 mRNA positive cells were morphologically assessed as macrophages or fibroblasts and myofibroblasts. In addition, expression of MMP-1 mRNA was detected in epithelial cells (E). TIMP-1 mRNA localised to the same regions as MMP-1 and MMP-3 mRNA but without epithelial staining (C). Positive staining for collagen I mRNA can be seen over subepithelial myofibroblasts and fibroblasts (D). (F) Sense hybridisation for procollagen I. Original magnification × 100 (dark field photographs and E) and × 200.
Figure 4 .
(A, B) The expression of matrix metalloproteinase (MMP)-1 mRNA in untreated coeliac disease (CD) epithelial cells is significantly increased per high power field and per total cells compared with controls and treated CD. (C) MMP-1 mRNA expression per positive cells is also increased in untreated CD compared with controls and treated CD. For further explanations, see fig 1.
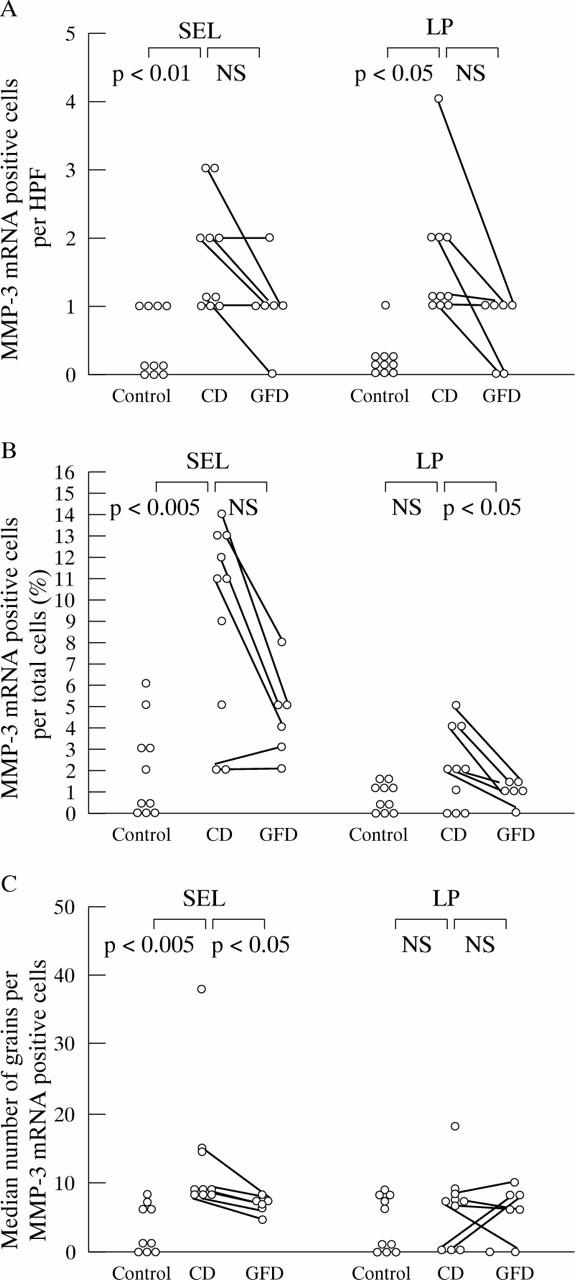
Figure 5 .
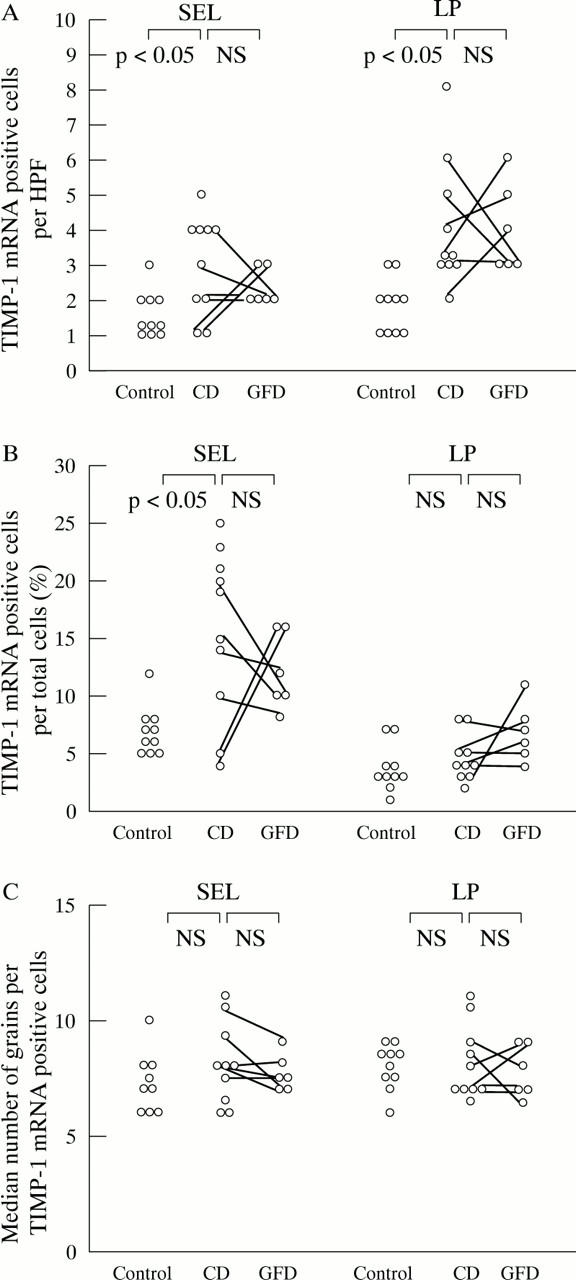
Expression of tissue inhibitor of metalloproteinases (TIMP)-1 mRNA is significantly increased in the subepithelial layer of biopsy specimens from patients with coeliac disease (CD) (as percentage of positive cells per total number of cells (B) as well as positive cells per high power field (HPF) (A)) and in the lamina propria (A) compared with controls. (C) TIMP-1 mRNA expression is not altered by the gluten free diet in CD biopsy specimens, as TIMP-1 mRNA expression per positive cell is also not increased in untreated CD compared with controls and treated CD. For further explanations, see fig 1.
Figure 6 .
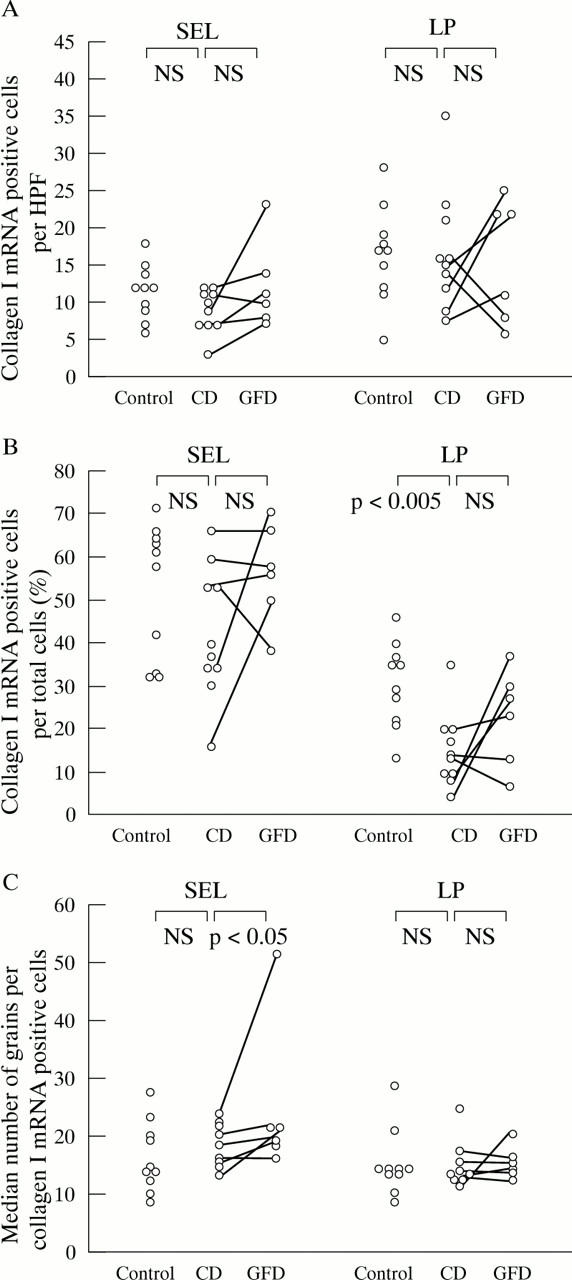
Expression of procollagen α1(I) mRNA in the duodenal mucosa of patients with active and treated CD and in uninflamed control tissue. Only the percentages of procollagen α1(I) mRNA positive cells per total cells are significantly reduced in CD biopsy specimens compared with controls. After a gluten free diet, procollagen α1(I) mRNA expression per positive cell in the subepithelial layer is increased compared with before the gluten free diet. For further explanations, see fig 1.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkedal-Hansen H. Proteolytic remodeling of extracellular matrix. Curr Opin Cell Biol. 1995 Oct;7(5):728–735. doi: 10.1016/0955-0674(95)80116-2. [DOI] [PubMed] [Google Scholar]
- Bossart R., Henry K., Booth C. C., Doe W. F. Subepithelial collagen in intestinal malabsorption. Gut. 1975 Jan;16(1):18–22. doi: 10.1136/gut.16.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombel J. F., Hällgren R., Engström-Laurent A., Rambaud J. C. Hyaluronic acid and type III procollagen peptide in jejunal perfusion fluid as markers of connective tissue turnover. Gastroenterology. 1989 Jan;96(1):68–73. doi: 10.1016/0016-5085(89)90765-8. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeClerck Y., Draper V., Parkman R. Clonal analysis of murine graft-vs-host disease. II. Leukokines that stimulate fibroblast proliferation and collagen synthesis in graft-vs. host disease. J Immunol. 1986 May 15;136(10):3549–3552. [PubMed] [Google Scholar]
- Garside P., Felstein M. V., Green E. A., Mowat A. M. The role of interferon alpha/beta in the induction of intestinal pathology in mice. Immunology. 1991 Oct;74(2):279–283. [PMC free article] [PubMed] [Google Scholar]
- Gifford G. E., Flick D. A. Tumour necrosis factor. Microbiol Sci. 1988 Apr;5(4):104–107. [PubMed] [Google Scholar]
- Goldberg G. I., Marmer B. L., Grant G. A., Eisen A. Z., Wilhelm S., He C. S. Human 72-kilodalton type IV collagenase forms a complex with a tissue inhibitor of metalloproteases designated TIMP-2. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8207–8211. doi: 10.1073/pnas.86.21.8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M. F., Diegelmann R. F., Elson C. O., Lindblad W. J., Gotschalk N., Gay S., Gay R. Collagen content and types in the intestinal strictures of Crohn's disease. Gastroenterology. 1988 Feb;94(2):257–265. doi: 10.1016/0016-5085(88)90411-8. [DOI] [PubMed] [Google Scholar]
- Graham M. F., Willey A., Adams J., Yager D., Diegelmann R. F. Interleukin 1 beta down-regulates collagen and augments collagenase expression in human intestinal smooth muscle cells. Gastroenterology. 1996 Feb;110(2):344–350. doi: 10.1053/gast.1996.v110.pm8566579. [DOI] [PubMed] [Google Scholar]
- Hahn U., Stallmach A., Hahn E. G., Riecken E. O. Basement membrane components are potent promoters of rat intestinal epithelial cell differentiation in vitro. Gastroenterology. 1990 Feb;98(2):322–335. doi: 10.1016/0016-5085(90)90821-h. [DOI] [PubMed] [Google Scholar]
- Halstensen T. S., Brandtzaeg P. Activated T lymphocytes in the celiac lesion: non-proliferative activation (CD25) of CD4+ alpha/beta cells in the lamina propria but proliferation (Ki-67) of alpha/beta and gamma/delta cells in the epithelium. Eur J Immunol. 1993 Feb;23(2):505–510. doi: 10.1002/eji.1830230231. [DOI] [PubMed] [Google Scholar]
- Hein J., Hahn E., Riecken E. O. Kollagentypen und mononukleäre Zellen in der Dünndarmschleimhaut: Lokalisation bei Gesunden und Patienten mit Sprue. Verh Dtsch Ges Inn Med. 1977 Apr 17;83:452–455. [PubMed] [Google Scholar]
- Herbst H., Wege T., Milani S., Pellegrini G., Orzechowski H. D., Bechstein W. O., Neuhaus P., Gressner A. M., Schuppan D. Tissue inhibitor of metalloproteinase-1 and -2 RNA expression in rat and human liver fibrosis. Am J Pathol. 1997 May;150(5):1647–1659. [PMC free article] [PubMed] [Google Scholar]
- Kontakou M., Przemioslo R. T., Sturgess R. P., Limb G. A., Ellis H. J., Day P., Ciclitira P. J. Cytokine mRNA expression in the mucosa of treated coeliac patients after wheat peptide challenge. Gut. 1995 Jul;37(1):52–57. doi: 10.1136/gut.37.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin K. E., Scott H., Hansen T., Paulsen G., Halstensen T. S., Fausa O., Thorsby E., Sollid L. M. Gliadin-specific, HLA-DQ(alpha 1*0501,beta 1*0201) restricted T cells isolated from the small intestinal mucosa of celiac disease patients. J Exp Med. 1993 Jul 1;178(1):187–196. doi: 10.1084/jem.178.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T., Spencer J. Evidence that activated mucosal T cells play a role in the pathogenesis of enteropathy in human small intestine. J Exp Med. 1988 Apr 1;167(4):1341–1349. doi: 10.1084/jem.167.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M., Glaichenhaus N., Gesnel M. C., Breathnach R. Epidermal growth factor and oncogenes induce transcription of the same cellular mRNA in rat fibroblasts. EMBO J. 1985 Jun;4(6):1435–1440. doi: 10.1002/j.1460-2075.1985.tb03799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Matthes H., Herbst H., Schuppan D., Stallmach A., Milani S., Stein H., Riecken E. O. Cellular localization of procollagen gene transcripts in inflammatory bowel diseases. Gastroenterology. 1992 Feb;102(2):431–442. doi: 10.1016/0016-5085(92)90087-f. [DOI] [PubMed] [Google Scholar]
- Matthes H., Stallmach A., Matthes B., Herbst H., Schuppan D., Riecken E. O. Hinweise für einen differenten Kollagenmetabolismus bei Morbus Crohn und Colitis ulcerosa. Med Klin (Munich) 1993 Apr 15;88(4):185–192. [PubMed] [Google Scholar]
- Mauviel A. Cytokine regulation of metalloproteinase gene expression. J Cell Biochem. 1993 Dec;53(4):288–295. doi: 10.1002/jcb.240530404. [DOI] [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Grappone C., Pellegrini G., Pinzani M., Casini A., Calabró A., Ciancio G., Stefanini F. Differential expression of matrix-metalloproteinase-1 and -2 genes in normal and fibrotic human liver. Am J Pathol. 1994 Mar;144(3):528–537. [PMC free article] [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Hahn E. G., Stein H. In situ hybridization for procollagen types I, III and IV mRNA in normal and fibrotic rat liver: evidence for predominant expression in nonparenchymal liver cells. Hepatology. 1989 Jul;10(1):84–92. doi: 10.1002/hep.1840100117. [DOI] [PubMed] [Google Scholar]
- Mowat A. M. Antibodies to IFN-gamma prevent immunologically mediated intestinal damage in murine graft-versus-host reaction. Immunology. 1989 Sep;68(1):18–23. [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., Hutton A. K., Garside P., Steel M. A role for interleukin-1 alpha in immunologically mediated intestinal pathology. Immunology. 1993 Sep;80(1):110–115. [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Reynolds J. J., Werb Z. Biosynthesis of tissue inhibitor of metalloproteinases by human fibroblasts in culture. Stimulation by 12-O-tetradecanoylphorbol 13-acetate and interleukin 1 in parallel with collagenase. J Biol Chem. 1985 Mar 10;260(5):3079–3083. [PubMed] [Google Scholar]
- Nilsen E. M., Lundin K. E., Krajci P., Scott H., Sollid L. M., Brandtzaeg P. Gluten specific, HLA-DQ restricted T cells from coeliac mucosa produce cytokines with Th1 or Th0 profile dominated by interferon gamma. Gut. 1995 Dec;37(6):766–776. doi: 10.1136/gut.37.6.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pender S. L., Breese E. J., Günther U., Howie D., Wathen N. C., Schuppan D., MacDonald T. T. Suppression of T cell-mediated injury in human gut by interleukin 10: role of matrix metalloproteinases. Gastroenterology. 1998 Sep;115(3):573–583. doi: 10.1016/s0016-5085(98)70136-2. [DOI] [PubMed] [Google Scholar]
- Pender S. L., Fell J. M., Chamow S. M., Ashkenazi A., MacDonald T. T. A p55 TNF receptor immunoadhesin prevents T cell-mediated intestinal injury by inhibiting matrix metalloproteinase production. J Immunol. 1998 Apr 15;160(8):4098–4103. [PubMed] [Google Scholar]
- Pender S. L., Tickle S. P., Docherty A. J., Howie D., Wathen N. C., MacDonald T. T. A major role for matrix metalloproteinases in T cell injury in the gut. J Immunol. 1997 Feb 15;158(4):1582–1590. [PubMed] [Google Scholar]
- Piguet P. F., Grau G. E., Allet B., Vassalli P. Tumor necrosis factor/cachectin is an effector of skin and gut lesions of the acute phase of graft-vs.-host disease. J Exp Med. 1987 Nov 1;166(5):1280–1289. doi: 10.1084/jem.166.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przemioslo R. T., Kontakou M., Nobili V., Ciclitira P. J. Raised pro-inflammatory cytokines interleukin 6 and tumour necrosis factor alpha in coeliac disease mucosa detected by immunohistochemistry. Gut. 1994 Oct;35(10):1398–1403. doi: 10.1136/gut.35.10.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch Dis Child. 1990 Aug;65(8):909–911. doi: 10.1136/adc.65.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarialho-Kere U. K., Vaalamo M., Puolakkainen P., Airola K., Parks W. C., Karjalainen-Lindsberg M. L. Enhanced expression of matrilysin, collagenase, and stromelysin-1 in gastrointestinal ulcers. Am J Pathol. 1996 Feb;148(2):519–526. [PMC free article] [PubMed] [Google Scholar]
- Schuppan D., Riecken E. O. Molecules of the extracellular matrix: potential role of collagens and glycoproteins in intestinal adaptation. Digestion. 1990;46 (Suppl 2):2–11. doi: 10.1159/000200360. [DOI] [PubMed] [Google Scholar]
- Whitelock J. M., Paine M. L., Gibbins J. R., Kefford R. F., O'Grady R. L. Multiple levels of post-transcriptional regulation of collagenase (matrix metalloproteinase 1) in an epithelial cell line. Immunol Cell Biol. 1993 Feb;71(Pt 1):39–47. doi: 10.1038/icb.1993.4. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991 May;5(8):2145–2154. [PubMed] [Google Scholar]