Abstract
Background—Polyamines are essential for cell growth. Dietary and probably gut bacterial derived polyamines contribute significantly to the polyamine body pool. Aims—To evaluate the influence of dietary, luminal polyamines on growth and development of different gastrointestinal organs in normally growing rats. Methods—Male suckling Wistar rats were randomly allocated to four treatment groups: polyamine deficient diet (PDD); PDD plus antibiotics (neomycin 2 g/kg and metronidazole 34 mg/kg); PDD plus polyamine supplementation at normal concentrations; or normal standard laboratory chow. After a six month feeding period 7-10 animals/group were sacrificed. Results—No differences in body weight gain, food consumption, or general behaviour could be observed between the four groups of animals. Feeding of PDD alone or PDD plus antibiotics resulted in a highly significant decrease in organ weight, protein content, and DNA content in small intestinal and colonic mucosa whereas no alterations were found in the liver. Conclusions—Long term feeding of polyamine deficient diets resulted in a significant hypoplasia of small intestinal and colonic mucosa. Dietary, luminal polyamines are important local factors for growth and the development of small intestinal and colonic mucosa.
Keywords: colon; gut; nutrition; ornithine decarboxylase; polyamines; polyamine deficient diet
Full Text
The Full Text of this article is available as a PDF (119.5 KB).
Figure 1 .
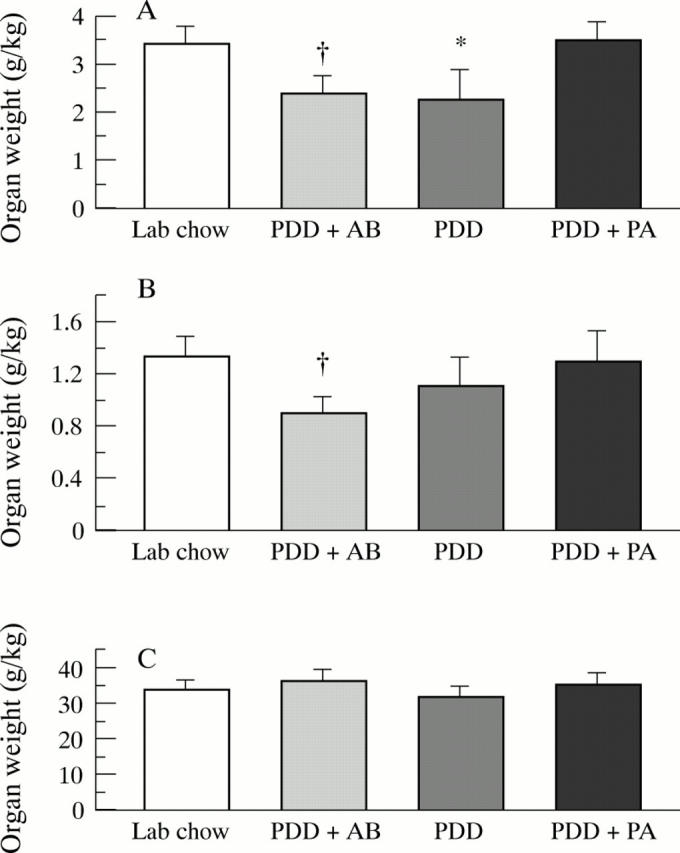
Organ weight of (A) small intestinal mucosa, (B) colonic mucosa, and (C) liver in rats (n=7-10) after a 26 week feeding period with either PDD, PDD + AB, PDD + PA, or standard laboratory chow. Significant differences versus PDD + PA: *p<0.01; †p<0.005.
Figure 2 .
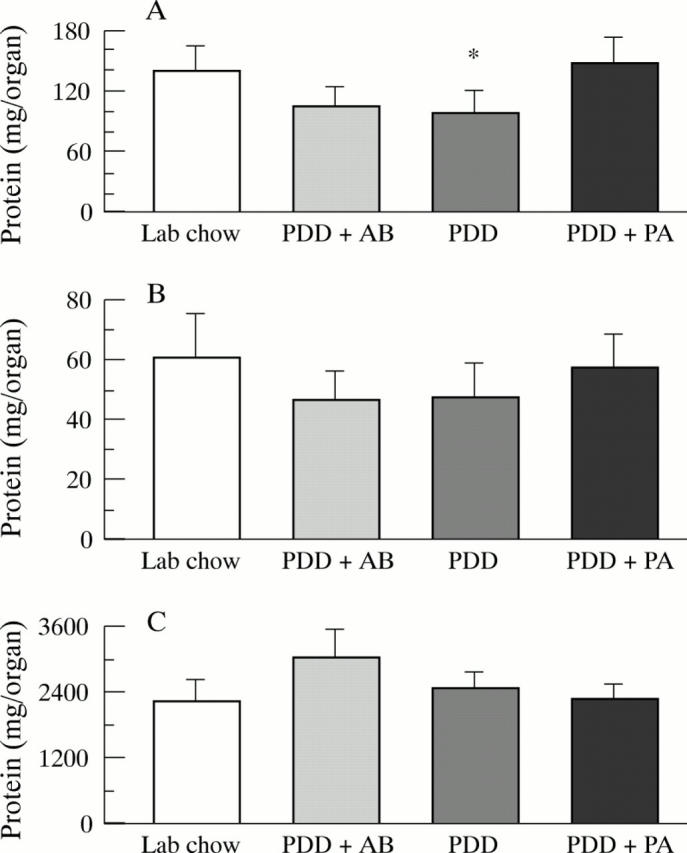
Protein content of (A) small intestinal mucosa, (B) colonic mucosa, and (C) liver in rats (n=7-10) after a 26 week feeding period with either PDD, PDD + AB, PDD + PA, or standard laboratory chow. Significant differences versus PDD + PA: *p<0.01.
Figure 3 .
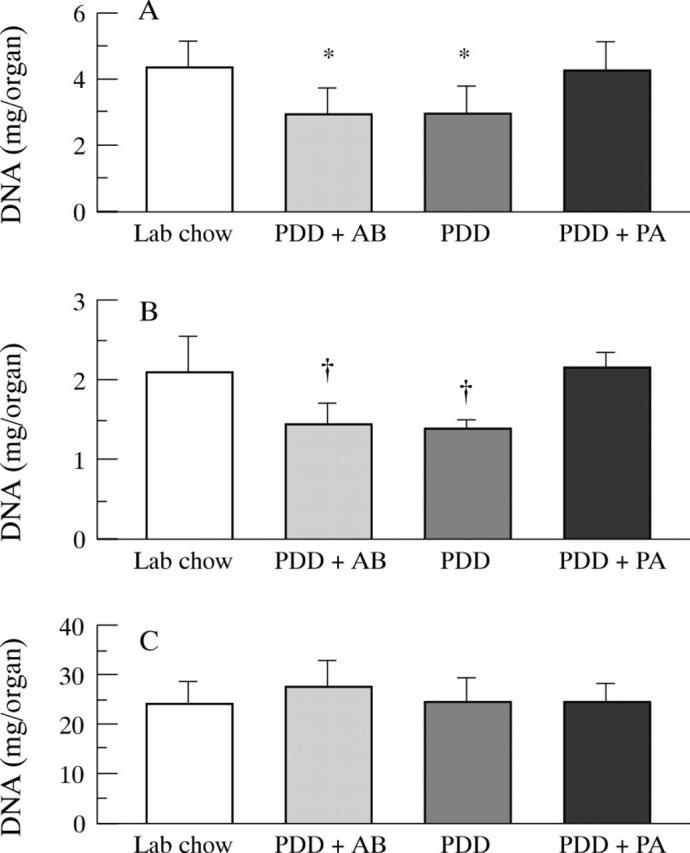
DNA content of (A) small intestinal mucosa, (B) colonic mucosa, and (C) liver in rats (n=7-10) after a 26 week feeding period with either PDD, PDD + AB, PDD + PA, or standard laboratory chow. Significant differences versus PDD + PA: *p<0.01; †p<0.005.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardocz S., Brown D. S., Grant G., Pusztai A. Luminal and basolateral polyamine uptake by rat small intestine stimulated to grow by Phaseolus vulgaris lectin phytohaemagglutinin in vivo. Biochim Biophys Acta. 1990 Apr 23;1034(1):46–52. doi: 10.1016/0304-4165(90)90151-l. [DOI] [PubMed] [Google Scholar]
- Bardócz S., Duguid T. J., Brown D. S., Grant G., Pusztai A., White A., Ralph A. The importance of dietary polyamines in cell regeneration and growth. Br J Nutr. 1995 Jun;73(6):819–828. doi: 10.1079/bjn19950087. [DOI] [PubMed] [Google Scholar]
- Bardócz S., Grant G., Brown D. S., Ewen S. W., Nevison I., Pusztai A. Polyamine metabolism and uptake during Phaseolus vulgaris lectin, PHA-induced growth of rat small intestine. Digestion. 1990;46 (Suppl 2):360–366. doi: 10.1159/000200409. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buts J. P., De Keyser N., Kolanowski J., Sokal E., Van Hoof F. Maturation of villus and crypt cell functions in rat small intestine. Role of dietary polyamines. Dig Dis Sci. 1993 Jun;38(6):1091–1098. doi: 10.1007/BF01295726. [DOI] [PubMed] [Google Scholar]
- Dorhout B., van Faassen A., van Beusekom C. M., Kingma A. W., de Hoog E., Nagel G. T., Karrenbeld A., Boersma E. R., Muskiet F. A. Oral administration of deuterium-labelled polyamines to sucking rat pups: luminal uptake, metabolic fate and effects on gastrointestinal maturation. Br J Nutr. 1997 Oct;78(4):639–654. doi: 10.1079/bjn19970180. [DOI] [PubMed] [Google Scholar]
- Dowling R. H. Cellular and molecular basis of intestinal and pancreatic adaptation. Scand J Gastroenterol Suppl. 1992;193:64–67. doi: 10.3109/00365529209096008. [DOI] [PubMed] [Google Scholar]
- Dowling R. H. Polyamines in intestinal adaptation and disease. Digestion. 1990;46 (Suppl 2):331–344. doi: 10.1159/000200406. [DOI] [PubMed] [Google Scholar]
- Dufour C., Dandrifosse G., Forget P., Vermesse F., Romain N., Lepoint P. Spermine and spermidine induce intestinal maturation in the rat. Gastroenterology. 1988 Jul;95(1):112–116. doi: 10.1016/0016-5085(88)90298-3. [DOI] [PubMed] [Google Scholar]
- Haarstad H., Winnberg A., Petersen H. Effects of a cholecystokinin-like peptide on DNA and polyamine synthesis in the rat pancreas. Scand J Gastroenterol. 1985 May;20(4):530–538. doi: 10.3109/00365528509089692. [DOI] [PubMed] [Google Scholar]
- Hessels J., Kingma A. W., Ferwerda H., Keij J., van den Berg G. A., Muskiet F. A. Microbial flora in the gastrointestinal tract abolishes cytostatic effects of alpha-difluoromethylornithine in vivo. Int J Cancer. 1989 Jun 15;43(6):1155–1164. doi: 10.1002/ijc.2910430632. [DOI] [PubMed] [Google Scholar]
- Kaouass M., Deloyer P., Wery I., Dandrifosse G. Analysis of structural and biochemical events occurring in the small intestine after dietary polyamine ingestion in suckling rats. Dig Dis Sci. 1996 Jul;41(7):1434–1444. doi: 10.1007/BF02088570. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Iseki K., Saitoh H., Miyazaki K. Uptake characteristics of polyamines into rat intestinal brush-border membrane. Biochim Biophys Acta. 1992 Mar 23;1105(1):177–183. doi: 10.1016/0005-2736(92)90177-n. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Löser C., Fitting T., Fölsch U. R. Importance of intracellular S-adenosylmethionine decarboxylase activity for the regulation of camostate-induced pancreatic polyamine metabolism and growth: in vivo effect of two novel S-adenosylmethionine decarboxylase inhibitors. Digestion. 1997;58(3):258–265. doi: 10.1159/000201452. [DOI] [PubMed] [Google Scholar]
- Löser C., Fölsch U. R., Cleffmann U., Nustede R., Creutzfeldt W. Role of ornithine decarboxylase and polyamines in camostate (Foy-305)-induced pancreatic growth in rats. Digestion. 1989;43(1-2):98–112. doi: 10.1159/000199867. [DOI] [PubMed] [Google Scholar]
- Löser C., Torff L., Fölsch U. R. Uptake of extracellular, dietary putrescine is an important regulatory mechanism of intracellular polyamine metabolism during camostate-induced pancreatic growth in rats. Dig Dis Sci. 1997 Mar;42(3):503–513. doi: 10.1023/a:1018882606579. [DOI] [PubMed] [Google Scholar]
- Löser C., Wunderlich U., Fölsch U. R. Reversed-phase liquid chromatographic separation and simultaneous fluorimetric detection of polyamines and their monoacetyl derivatives in human and animal urine, serum and tissue samples: an improved, rapid and sensitive method for routine application. J Chromatogr. 1988 Sep 9;430(2):249–262. doi: 10.1016/s0378-4347(00)83160-6. [DOI] [PubMed] [Google Scholar]
- Matsui I., Wiegand L., Pegg A. E. Properties of spermidine N-acetyltransferase from livers of rats treated with carbon tetrachloride and its role in the conversion of spermidine into putrescine. J Biol Chem. 1981 Mar 10;256(5):2454–2459. [PubMed] [Google Scholar]
- Milovic V., Stein J., Piiper A., Gerhard R., Zeuzem S., Caspary W. F. Characterization of putrescine transport across the intestinal epithelium: study using isolated brush border and basolateral membrane vesicles of the enterocyte. Eur J Clin Invest. 1995 Feb;25(2):97–105. doi: 10.1111/j.1365-2362.1995.tb01533.x. [DOI] [PubMed] [Google Scholar]
- Osborne D. L., Seidel E. R. Gastrointestinal luminal polyamines: cellular accumulation and enterohepatic circulation. Am J Physiol. 1990 Apr;258(4 Pt 1):G576–G584. doi: 10.1152/ajpgi.1990.258.4.G576. [DOI] [PubMed] [Google Scholar]
- Osborne D. L., Seidel E. R. Microflora-derived polyamines modulate obstruction-induced colonic mucosal hypertrophy. Am J Physiol. 1989 Jun;256(6 Pt 1):G1049–G1057. doi: 10.1152/ajpgi.1989.256.6.G1049. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988 Feb 15;48(4):759–774. [PubMed] [Google Scholar]
- Quemener V., Blanchard Y., Chamaillard L., Havouis R., Cipolla B., Moulinoux J. P. Polyamine deprivation: a new tool in cancer treatment. Anticancer Res. 1994 Mar-Apr;14(2A):443–448. [PubMed] [Google Scholar]
- Sarhan S., Knodgen B., Seiler N. The gastrointestinal tract as polyamine source for tumor growth. Anticancer Res. 1989 Jan-Feb;9(1):215–223. [PubMed] [Google Scholar]
- Sarhan S., Knödgen B., Seiler N. Polyamine deprivation, malnutrition and tumor growth. Anticancer Res. 1992 Mar-Apr;12(2):457–466. [PubMed] [Google Scholar]
- Seiler N., Delcros J. G., Moulinoux J. P. Polyamine transport in mammalian cells. An update. Int J Biochem Cell Biol. 1996 Aug;28(8):843–861. doi: 10.1016/1357-2725(96)00021-0. [DOI] [PubMed] [Google Scholar]
- Seiler N. Polyamine metabolism. Digestion. 1990;46 (Suppl 2):319–330. doi: 10.1159/000200405. [DOI] [PubMed] [Google Scholar]
- Seiler N., Sarhan S., Grauffel C., Jones R., Knödgen B., Moulinoux J. P. Endogenous and exogenous polyamines in support of tumor growth. Cancer Res. 1990 Aug 15;50(16):5077–5083. [PubMed] [Google Scholar]


