Abstract
Background—A familial defect in intestinal barrier function has been found in Crohn's disease. Aim—To investigate possible genetic and environmental influences on this barrier defect by studying intestinal permeability in both relatives and spouses of patients with Crohn's disease. Subjects—The study included 39 patients with Crohn's disease, 34 healthy first degree relatives, and 22 spouses. Twenty nine healthy volunteers served as controls. Methods—Intestinal permeability was assessed as the lactulose:mannitol ratio in five hour urinary excretion after oral load, both before (baseline) and after ingestion of acetylsalicylic acid. The permeability response represents the difference between the two tests. A ratio above the 95th percentile for controls was classified as abnormal. Results—Baseline permeability was higher in patients and spouses than in controls. An abnormal baseline permeability was seen in 36% of the patients, 23% of the spouses, 18% of the relatives, and 3% of the controls. After ingestion of acetylsalicylic acid, permeability increased significantly in all groups. Relatives were similar to patients with regard to permeability after exposure to acetylsalicylic acid, whereas spouses were similar to controls. The proportions with an abnormal permeability response to acetylsalicylic acid were 32% in patients, 14% in spouses, 41% in relatives, and 3% in controls. Conclusion—The findings suggest that baseline permeability is determined by environmental factors, whereas permeability provoked by acetylsalicylic acid is a function of the genetically determined state of the mucosal barrier, and support the notion that environmental and hereditary factors interact in the pathogenesis of Crohn's disease.
Keywords: acetylsalicylic acid; environment; genetics; inflammatory bowel disease; lactulose; mannitol
Full Text
The Full Text of this article is available as a PDF (128.4 KB).
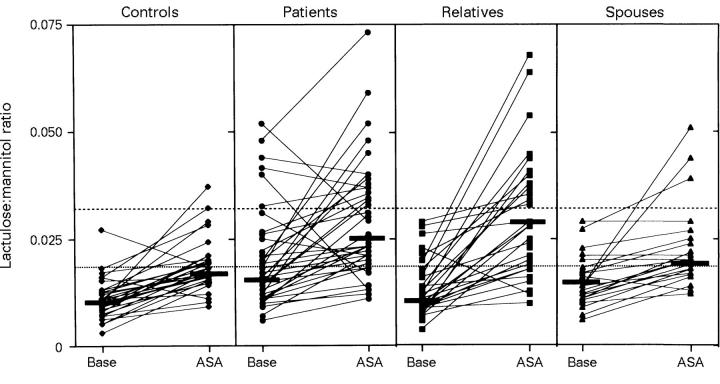
Figure 1 .
Paired scattergrams of lactulose:mannitol (L/M) ratio in five hour urine samples before (Base) and after oral ingestion of 2 × 1.25 g acetylsalicylic acid (ASA) in the various study groups. The subjects tested were healthy controls (n = 29), Crohn's disease patients (n = 39), first degree relatives (n = 34), and spouses (n = 22). Solid lines indicate the median L/M ratios in the groups. The upper limit of normality (95th percentile in the control group) at baseline (0.0195) is indicated by the dotted line and the upper limit after ASA (0.032) is indicated by the dashed line. At baseline the median L/M ratio was increased in patients and spouses as compared with controls (p<0.05, Kruskal-Wallis). After intake of ASA the L/M ratio was higher in both patients and relatives than in controls (p<0.05, Kruskal-Wallis), but not in spouses. Note the marked increase in permeability in a large subgroup of the relatives after ingestion of ASA.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahrenstedt O., Knutson L., Nilsson B., Nilsson-Ekdahl K., Odlind B., Hällgren R. Enhanced local production of complement components in the small intestines of patients with Crohn's disease. N Engl J Med. 1990 May 10;322(19):1345–1349. doi: 10.1056/NEJM199005103221903. [DOI] [PubMed] [Google Scholar]
- Ainsworth M., Eriksen J., Rasmussen J. W., Schaffalitzky de Muckadell O. B. Intestinal permeability of 51Cr-labelled ethylenediaminetetraacetic acid in patients with Crohn's disease and their healthy relatives. Scand J Gastroenterol. 1989 Oct;24(8):993–998. doi: 10.3109/00365528909089246. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Batt R., Catt S., Macpherson A., Maxton D., Menzies I. S. Evaluation of differential disaccharide excretion in urine for non-invasive investigation of altered intestinal disaccharidase activity caused by alpha-glucosidase inhibition, primary hypolactasia, and coeliac disease. Gut. 1996 Sep;39(3):374–381. doi: 10.1136/gut.39.3.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I., MacPherson A., Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995 May;108(5):1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- Brodie D. A., Tate C. L., Hooke K. F. Aspirin: intestinal damage in rats. Science. 1970 Oct 9;170(3954):183–185. doi: 10.1126/science.170.3954.183. [DOI] [PubMed] [Google Scholar]
- Davies N. M., Corrigan B. W., Jamali F. Sucrose urinary excretion in the rat measured using a simple assay: a model of gastroduodenal permeability. Pharm Res. 1995 Nov;12(11):1733–1736. doi: 10.1023/a:1016221923679. [DOI] [PubMed] [Google Scholar]
- Gatti G., Barzaghi N., Attardo Parrinello G., Vitiello B., Perucca E. Pharmacokinetics of salicylic acid following administration of aspirin tablets and three different forms of soluble aspirin in normal subjects. Int J Clin Pharmacol Res. 1989;9(6):385–389. [PubMed] [Google Scholar]
- Hermiston M. L., Gordon J. I. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995 Nov 17;270(5239):1203–1207. doi: 10.1126/science.270.5239.1203. [DOI] [PubMed] [Google Scholar]
- Hilsden R. J., Meddings J. B., Sutherland L. R. Intestinal permeability changes in response to acetylsalicylic acid in relatives of patients with Crohn's disease. Gastroenterology. 1996 May;110(5):1395–1403. doi: 10.1053/gast.1996.v110.pm8613043. [DOI] [PubMed] [Google Scholar]
- Hollander D. Permeability in Crohn's disease: altered barrier functions in healthy relatives? Gastroenterology. 1993 Jun;104(6):1848–1851. doi: 10.1016/0016-5085(93)90668-3. [DOI] [PubMed] [Google Scholar]
- Hollander D. The intestinal permeability barrier. A hypothesis as to its regulation and involvement in Crohn's disease. Scand J Gastroenterol. 1992 Sep;27(9):721–726. doi: 10.3109/00365529209011172. [DOI] [PubMed] [Google Scholar]
- Hollander D., Vadheim C. M., Brettholz E., Petersen G. M., Delahunty T., Rotter J. I. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann Intern Med. 1986 Dec;105(6):883–885. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- Kynaston J. A., Fleming S. C., Laker M. F., Pearson A. D. Simultaneous quantification of mannitol, 3-O-methyl glucose, and lactulose in urine by HPLC with pulsed electrochemical detection, for use in studies of intestinal permeability. Clin Chem. 1993 Mar;39(3):453–456. [PubMed] [Google Scholar]
- Lindberg E., Söderholm J. D., Olaison G., Tysk C., Järnerot G. Intestinal permeability to polyethylene glycols in monozygotic twins with Crohn's disease. Scand J Gastroenterol. 1995 Aug;30(8):780–783. doi: 10.3109/00365529509096327. [DOI] [PubMed] [Google Scholar]
- Madara J. L., Parkos C., Colgan S., Nusrat A., Atisook K., Kaoutzani P. The movement of solutes and cells across tight junctions. Ann N Y Acad Sci. 1992;664:47–60. doi: 10.1111/j.1749-6632.1992.tb39748.x. [DOI] [PubMed] [Google Scholar]
- Marteau P., Colombel J. F., Nemeth J., Vaerman J. P., Dive J. C., Rambaud J. C. Immunological study of histologically non-involved jejunum during Crohn's disease: evidence for reduced in vivo secretion of secretory IgA. Clin Exp Immunol. 1990 May;80(2):196–201. doi: 10.1111/j.1365-2249.1990.tb05233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May G. R., Sutherland L. R., Meddings J. B. Is small intestinal permeability really increased in relatives of patients with Crohn's disease? Gastroenterology. 1993 Jun;104(6):1627–1632. doi: 10.1016/0016-5085(93)90638-s. [DOI] [PubMed] [Google Scholar]
- Meddings J. B., Sutherland L. R., Byles N. I., Wallace J. L. Sucrose: a novel permeability marker for gastroduodenal disease. Gastroenterology. 1993 Jun;104(6):1619–1626. doi: 10.1016/0016-5085(93)90637-r. [DOI] [PubMed] [Google Scholar]
- Morson B. S. Histopathology of Crohn's disease. Proc R Soc Med. 1968 Jan;61(1):79–81. [PMC free article] [PubMed] [Google Scholar]
- Muir N., Nichols J. D., Clifford J. M., Stillings M. R., Hoare R. C. The influence of dosage form on aspirin kinetics: implications for acute cardiovascular use. Curr Med Res Opin. 1997;13(10):547–553. doi: 10.1185/03007999709113328. [DOI] [PubMed] [Google Scholar]
- Munkholm P., Langholz E., Hollander D., Thornberg K., Orholm M., Katz K. D., Binder V. Intestinal permeability in patients with Crohn's disease and ulcerative colitis and their first degree relatives. Gut. 1994 Jan;35(1):68–72. doi: 10.1136/gut.35.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaison G., Sjödahl R., Tagesson C. Abnormal intestinal permeability in Crohn's disease. A possible pathogenic factor. Scand J Gastroenterol. 1990 Apr;25(4):321–328. doi: 10.3109/00365529009095493. [DOI] [PubMed] [Google Scholar]
- Olaison G., Sjödahl R., Tagesson C. Decreased gastrointestinal absorption of peroral polyethyleneglycols (PEG 1000) in Crohn's disease. A sign of jejunal abnormality. Acta Chir Scand. 1987;153(5-6):373–377. [PubMed] [Google Scholar]
- Peeters M., Geypens B., Claus D., Nevens H., Ghoos Y., Verbeke G., Baert F., Vermeire S., Vlietinck R., Rutgeerts P. Clustering of increased small intestinal permeability in families with Crohn's disease. Gastroenterology. 1997 Sep;113(3):802–807. doi: 10.1016/s0016-5085(97)70174-4. [DOI] [PubMed] [Google Scholar]
- Peeters M., Ghoos Y., Maes B., Hiele M., Geboes K., Vantrappen G., Rutgeerts P. Increased permeability of macroscopically normal small bowel in Crohn's disease. Dig Dis Sci. 1994 Oct;39(10):2170–2176. doi: 10.1007/BF02090367. [DOI] [PubMed] [Google Scholar]
- Pera A., Bellando P., Caldera D., Ponti V., Astegiano M., Barletti C., David E., Arrigoni A., Rocca G., Verme G. Colonoscopy in inflammatory bowel disease. Diagnostic accuracy and proposal of an endoscopic score. Gastroenterology. 1987 Jan;92(1):181–185. [PubMed] [Google Scholar]
- Russel M. G., Stockbrügger R. W. Epidemiology of inflammatory bowel disease: an update. Scand J Gastroenterol. 1996 May;31(5):417–427. doi: 10.3109/00365529609006759. [DOI] [PubMed] [Google Scholar]
- Ruttenberg D., Young G. O., Wright J. P., Isaacs S. PEG-400 excretion in patients with Crohn's disease, their first-degree relatives, and healthy volunteers. Dig Dis Sci. 1992 May;37(5):705–708. doi: 10.1007/BF01296426. [DOI] [PubMed] [Google Scholar]
- Sachar D. B. Crohn's disease: a family affair. Gastroenterology. 1996 Sep;111(3):813–815. doi: 10.1053/gast.1996.v111.agast961110813. [DOI] [PubMed] [Google Scholar]
- Satsangi J., Jewell D. P., Bell J. I. The genetics of inflammatory bowel disease. Gut. 1997 May;40(5):572–574. doi: 10.1136/gut.40.5.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasundaram S., Hayllar H., Rafi S., Wrigglesworth J. M., Macpherson A. J., Bjarnason I. The biochemical basis of non-steroidal anti-inflammatory drug-induced damage to the gastrointestinal tract: a review and a hypothesis. Scand J Gastroenterol. 1995 Apr;30(4):289–299. doi: 10.3109/00365529509093280. [DOI] [PubMed] [Google Scholar]
- Somasundaram S., Rafi S., Hayllar J., Sigthorsson G., Jacob M., Price A. B., Macpherson A., Mahmod T., Scott D., Wrigglesworth J. M. Mitochondrial damage: a possible mechanism of the "topical" phase of NSAID induced injury to the rat intestine. Gut. 1997 Sep;41(3):344–353. doi: 10.1136/gut.41.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teahon K., Smethurst P., Levi A. J., Menzies I. S., Bjarnason I. Intestinal permeability in patients with Crohn's disease and their first degree relatives. Gut. 1992 Mar;33(3):320–323. doi: 10.1136/gut.33.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis S., Menzies I. Intestinal permeability: functional assessment and significance. Clin Sci (Lond) 1992 May;82(5):471–488. doi: 10.1042/cs0820471. [DOI] [PubMed] [Google Scholar]
- Tysk C., Lindberg E., Järnerot G., Flodérus-Myrhed B. Ulcerative colitis and Crohn's disease in an unselected population of monozygotic and dizygotic twins. A study of heritability and the influence of smoking. Gut. 1988 Jul;29(7):990–996. doi: 10.1136/gut.29.7.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. L. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology. 1997 Mar;112(3):1000–1016. doi: 10.1053/gast.1997.v112.pm9041264. [DOI] [PubMed] [Google Scholar]
- Yacyshyn B. R., Meddings J. B. CD45RO expression on circulating CD19+ B cells in Crohn's disease correlates with intestinal permeability. Gastroenterology. 1995 Jan;108(1):132–137. doi: 10.1016/0016-5085(95)90017-9. [DOI] [PubMed] [Google Scholar]