Abstract
Background—Hepatic stellate cells play a key role in the pathogenesis of hepatic fibrosis. Aims—To examine the inhibitory effect of oestradiol on stellate cell activation. Methods—In vivo, hepatic fibrosis was induced in rats by dimethylnitrosamine or pig serum. In vitro, rat stellate cells were activated by contact with plastic dishes resulting in their transformation into myofibroblast-like cells. Results—In the dimethylnitrosamine and pig serum models, treatment with oestradiol at gestation related doses resulted in a dose dependent suppression of hepatic fibrosis with restored content of hepatic retinyl palmitate, reduced collagen content, lower areas of stellate cells which express α smooth muscle actin (α-SMA) and desmin, and lower procollagen type I and III mRNA levels in the liver. In cultured stellate cells, oestradiol inhibited type I collagen production, α-SMA expression, and cell proliferation. These findings suggest that oestradiol is a potent inhibitor of stellate cell transformation. Conclusion—The antifibrogenic role of oestradiol in the liver may contribute to the sex associated differences in the progression from hepatic fibrosis to cirrhosis.
Keywords: hepatic stellate cells; hepatic fibrosis; oestradiol; α smooth muscle actin; retinyl palmitate
Full Text
The Full Text of this article is available as a PDF (288.1 KB).
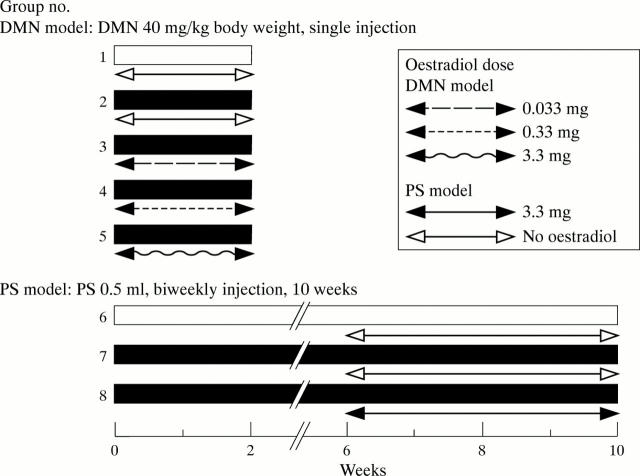
Figure 1 .
Experimental schedule in dimethylnitrosamine (DMN) and pig serum (PS) treated rats. Twenty five male Wistar rats were used for the DMN model and 15 for the PS model of hepatic fibrosis (eight groups of five each).
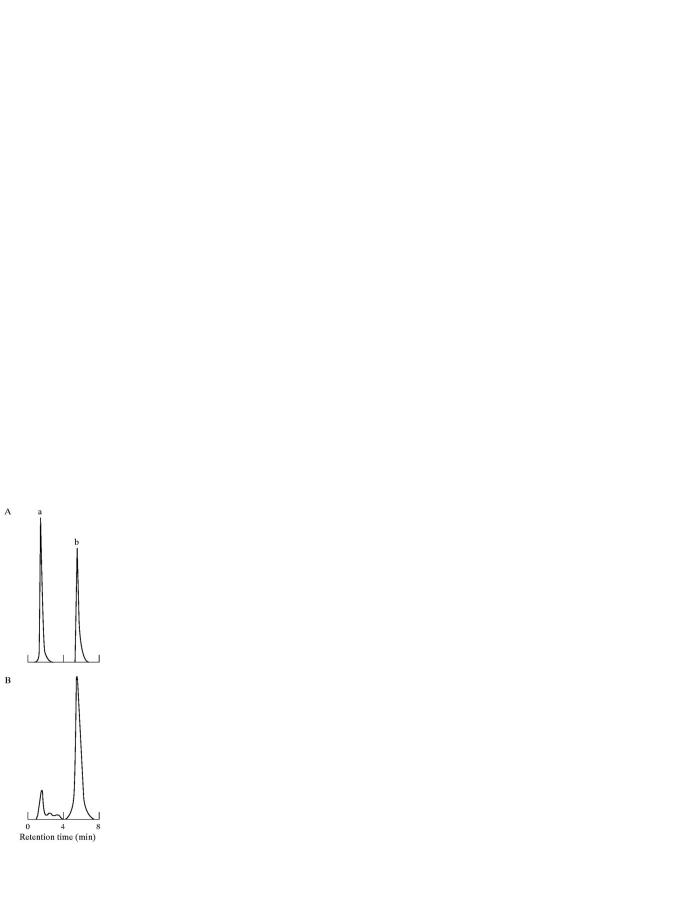
Figure 2 .
Representative HPLC chromatograms of (A) retinyl acetate and retinyl palmitate (RP) standards; and (B) a rat liver extract. Peaks: a, retinyl acetate (retention time, 1.9 minutes); b, RP, internal standard (retention time, 6.7 minutes).
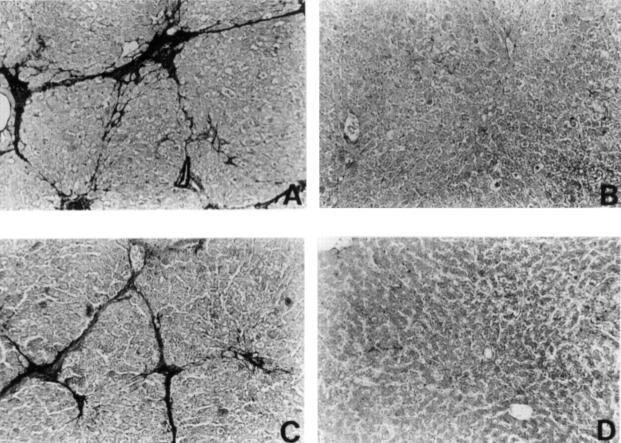
Figure 3 .
Reaction of representative liver sections with antibody to α smooth muscle actin (α-SMA). (A) Group 2; (B) Group 5; (C) Group 7; (D) Group 8. Original magnification ×60.
Figure 4 .
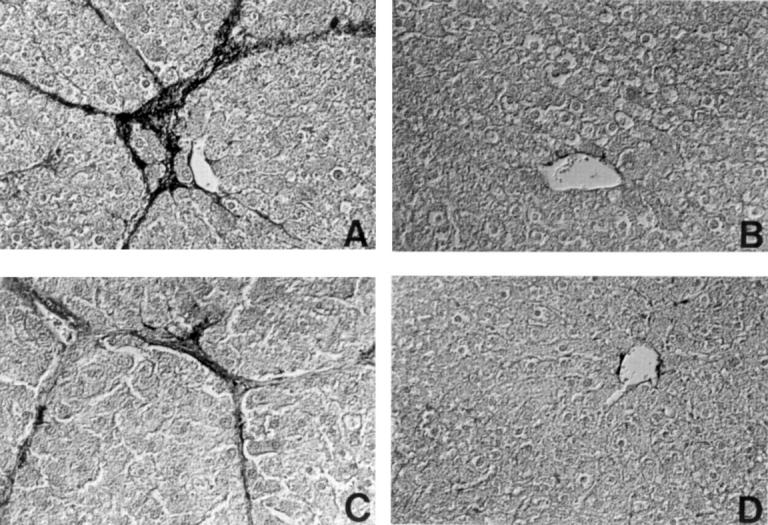
Reaction of representative liver sections with an antibody to desmin. (A) Group 2; (B) Group 5; (C) Group 7; (D) Group 8. Liver sections were photographed using a differential interference contrast microscope. Original magnification ×90.
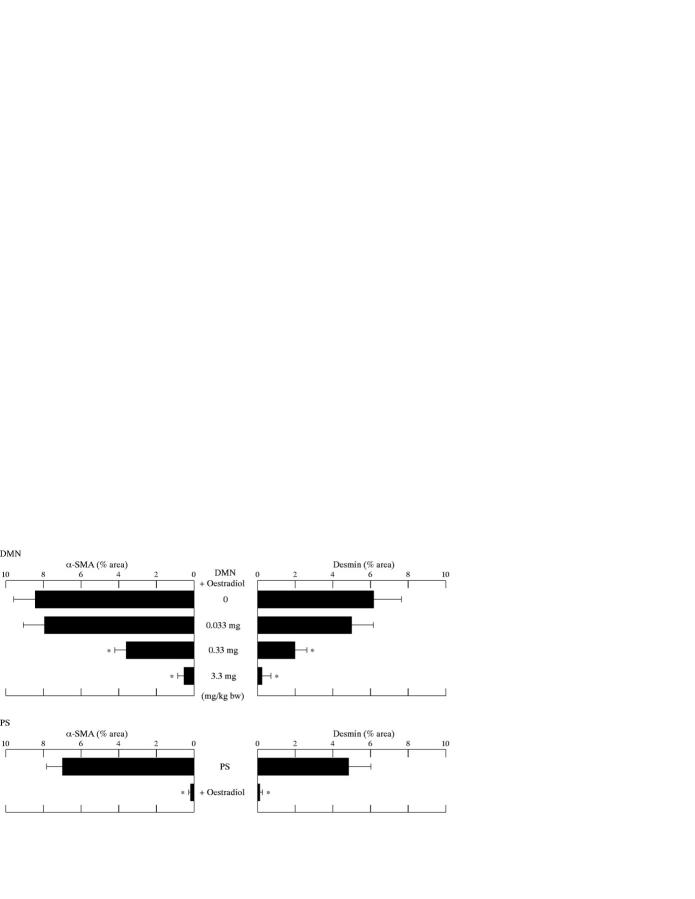
Figure 5 .
Percentage areas of α smooth muscle actin (α-SMA) and desmin positive cells after treatment with varying doses of oestradiol in the dimethylnitrosamine (DMN) and pig serum (PS) models. The mean value of α-SMA or desmin positive cells in six ocular fields (magnification ×40) per specimen was assessed as percentage area of α-SMA or desmin positive cells in six ocular fields at 40× magnification with five control rat livers. The area was measured using microcomputer based image analysis. *p<0.05 compared with rats treated with the same reagent (DMN or PS) but not given oestradiol.
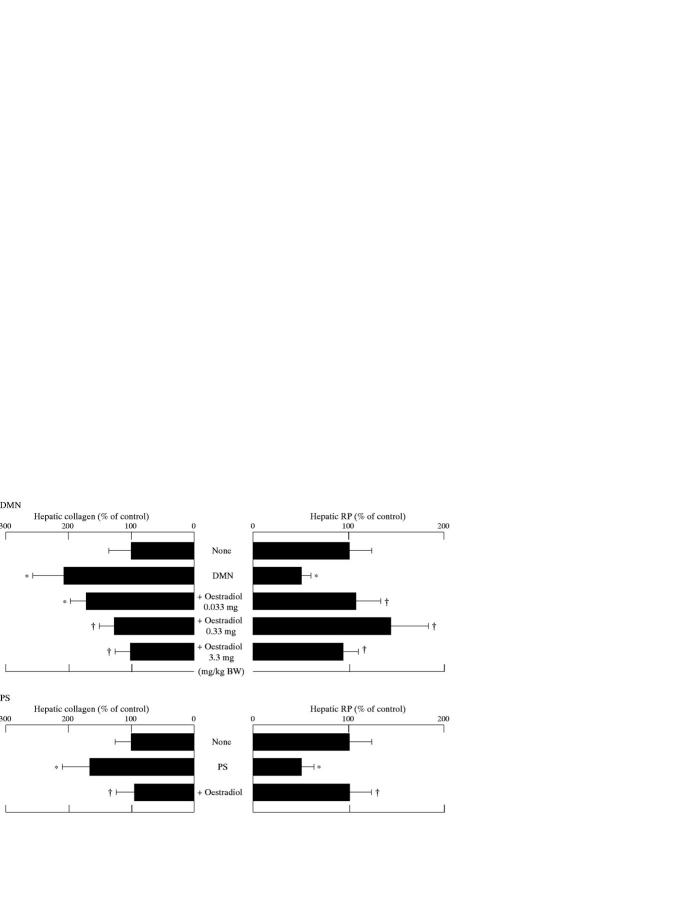
Figure 6 .
Effects of oestradiol on hepatic collagen and retinyl palmitate (RP) content in rats treated with dimethylnitrosamine (DMN) or pig serum (PS). All results are expressed as percentages (mean (SD)) of control values: in the DMN model, 765 (267) µg collagen/100 mg liver and 206 (34) IU RP/g liver; in the PS model, 796 (231) µg collagen/100 mg liver and 266 (36) IU RP/g liver. *p<0.05 compared with controls; †p<0.05 compared with rats treated with the same reagent (DMN or PS) but not given oestradiol.
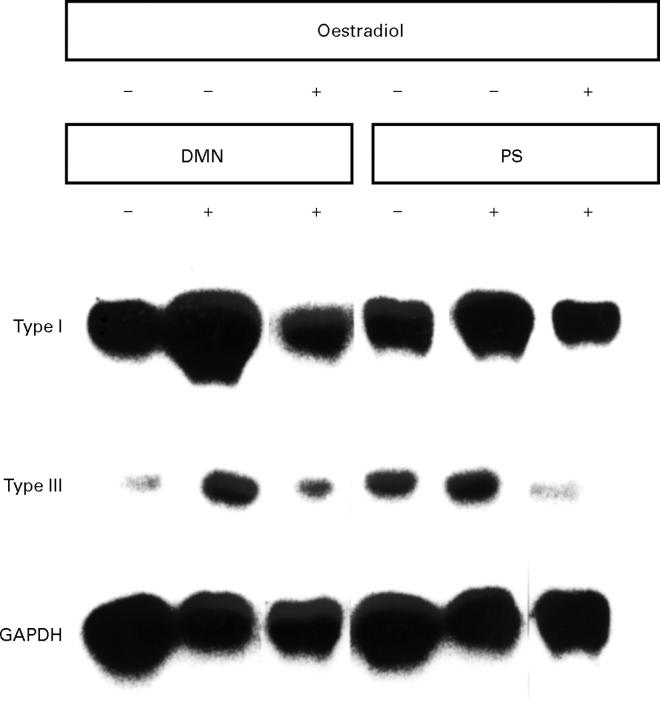
Figure 7 .
Northern blot analysis undertaken with extracts from livers of control rats or dimethylnitrosamine (DMN) and pig serum (PS) treated rats in the presence or absence of oestradiol from three separate experiments.
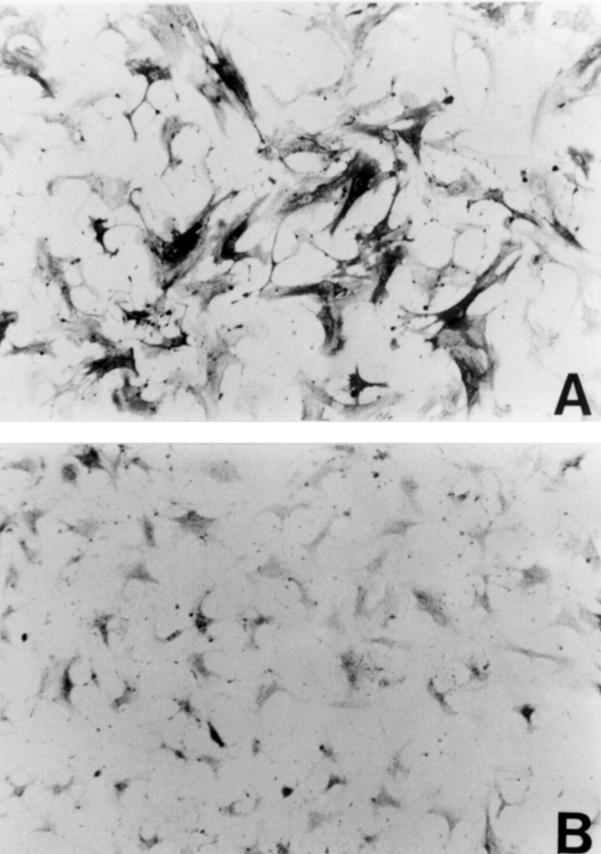
Figure 8 .
Reaction of cultured rat hepatic stellate cells with antibody to α smooth muscle actin (α-SMA). Cells were incubated in the (A) absence or (B) presence of oestradiol (10−6 M) for four days. Original magnification ×200.
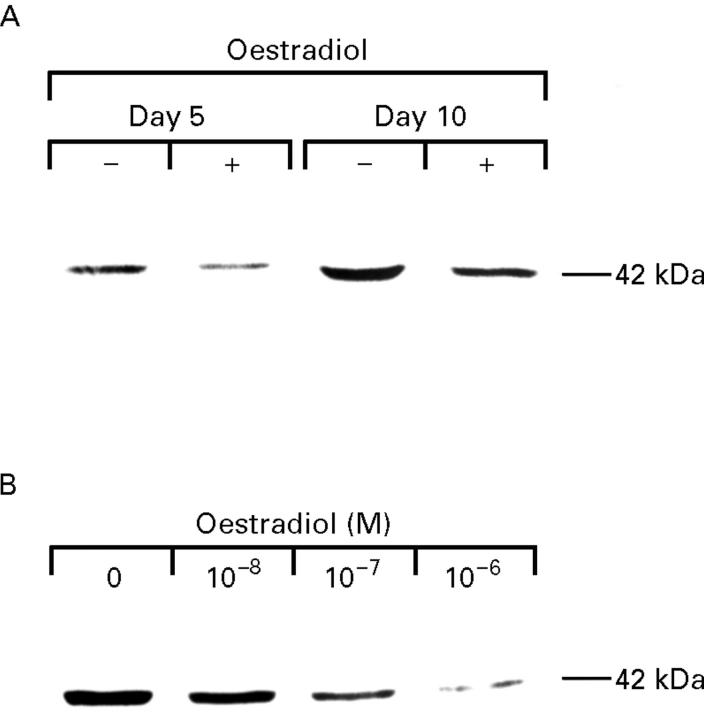
Figure 9 .
Effect of oestradiol on α smooth muscle actin (α-SMA) expression in cultured rat hepatic stellate cells. (A) Cells cultured for an additional three (day 5) or eight (day 10) days in the presence (+) or absence (−) of oestradiol (10−7 M). (B) Cells cultured for an additional eight days in the presence of oestradiol (10−8-10−6 M).
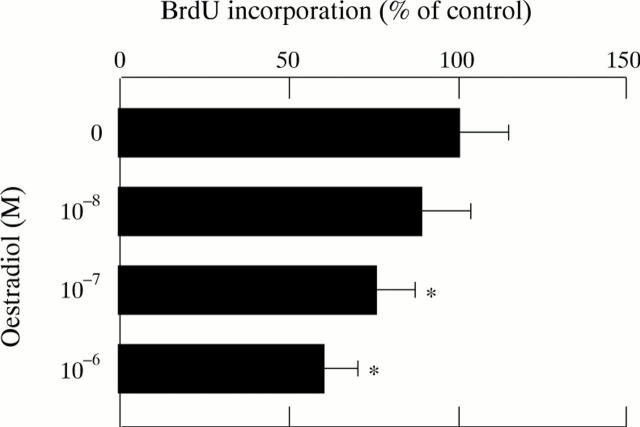
Figure 10 .
Effect of oestradiol on DNA synthesis in cultured rat hepatic stellate cells. Cells were incubated in the presence or absence of oestradiol (10−8-10−6 M) for 24 hours before being labelled with bromodeoxyuridine (BrdU) for 24 hours. Results expressed as percentages (SD) (n=4) of control value. *p<0.05 compared with controls.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baroni G. S., D'Ambrosio L., Curto P., Casini A., Mancini R., Jezequel A. M., Benedetti A. Interferon gamma decreases hepatic stellate cell activation and extracellular matrix deposition in rat liver fibrosis. Hepatology. 1996 May;23(5):1189–1199. doi: 10.1002/hep.510230538. [DOI] [PubMed] [Google Scholar]
- Beldekas J. C., Gerstenfeld L., Sonenshein G. E., Franzblau C. Cell density and estradiol modulation of procollagen type III in cultured calf smooth muscle cells. J Biol Chem. 1982 Oct 25;257(20):12252–12256. [PubMed] [Google Scholar]
- Chu M. L., Weil D., de Wet W., Bernard M., Sippola M., Ramirez F. Isolation of cDNA and genomic clones encoding human pro-alpha 1 (III) collagen. Partial characterization of the 3' end region of the gene. J Biol Chem. 1985 Apr 10;260(7):4357–4363. [PubMed] [Google Scholar]
- Czaja M. J., Weiner F. R., Takahashi S., Giambrone M. A., van der Meide P. H., Schellekens H., Biempica L., Zern M. A. Gamma-interferon treatment inhibits collagen deposition in murine schistosomiasis. Hepatology. 1989 Nov;10(5):795–800. doi: 10.1002/hep.1840100508. [DOI] [PubMed] [Google Scholar]
- Eagon P. K., Porter L. E., Francavilla A., DiLeo A., Van Thiel D. H. Estrogen and androgen receptors in liver: their role in liver disease and regeneration. Semin Liver Dis. 1985 Feb;5(1):59–69. doi: 10.1055/s-2008-1041758. [DOI] [PubMed] [Google Scholar]
- FENNELL R. H., Jr CHRONIC LIVER DISEASE INDUCED IN RATS BY REPEATED ANAPHYLACTIC SHOCK. Am J Pathol. 1965 Aug;47:173–182. [PMC free article] [PubMed] [Google Scholar]
- Fox H. S., Bond B. L., Parslow T. G. Estrogen regulates the IFN-gamma promoter. J Immunol. 1991 Jun 15;146(12):4362–4367. [PubMed] [Google Scholar]
- Friedman S. L. Cellular sources of collagen and regulation of collagen production in liver. Semin Liver Dis. 1990 Feb;10(1):20–29. doi: 10.1055/s-2008-1040454. [DOI] [PubMed] [Google Scholar]
- Friedman S. L., Roll F. J., Boyles J., Arenson D. M., Bissell D. M. Maintenance of differentiated phenotype of cultured rat hepatic lipocytes by basement membrane matrix. J Biol Chem. 1989 Jun 25;264(18):10756–10762. [PubMed] [Google Scholar]
- Gressner A. M., Bachem M. G. Cellular sources of noncollagenous matrix proteins: role of fat-storing cells in fibrogenesis. Semin Liver Dis. 1990 Feb;10(1):30–46. doi: 10.1055/s-2008-1040455. [DOI] [PubMed] [Google Scholar]
- Gressner A. M., Zerbe O. Kupffer cell-mediated induction of synthesis and secretion of proteoglycans by rat liver fat-storing cells in culture. J Hepatol. 1987 Dec;5(3):299–310. doi: 10.1016/s0168-8278(87)80036-3. [DOI] [PubMed] [Google Scholar]
- Gustafsson J. A., Mode A., Norstedt G., Skett P. Sex steroid induced changes in hepatic enzymes. Annu Rev Physiol. 1983;45:51–60. doi: 10.1146/annurev.ph.45.030183.000411. [DOI] [PubMed] [Google Scholar]
- Hendriks H. F., Verhoofstad W. A., Brouwer A., de Leeuw A. M., Knook D. L. Perisinusoidal fat-storing cells are the main vitamin A storage sites in rat liver. Exp Cell Res. 1985 Sep;160(1):138–149. doi: 10.1016/0014-4827(85)90243-5. [DOI] [PubMed] [Google Scholar]
- Jézéquel A. M., Mancini R., Rinaldesi M. L., Macarri G., Venturini C., Orlandi F. A morphological study of the early stages of hepatic fibrosis induced by low doses of dimethylnitrosamine in the rat. J Hepatol. 1987 Oct;5(2):174–181. doi: 10.1016/s0168-8278(87)80570-6. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Buck M., Houglum K., Chojkier M. Activation of hepatic stellate cells by TGF alpha and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest. 1995 Nov;96(5):2461–2468. doi: 10.1172/JCI118304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo M. A., Sato M., Lieber C. S. Effect of hepatic vitamin A depletion on the liver in humans and rats. Gastroenterology. 1983 Mar;84(3):562–572. [PubMed] [Google Scholar]
- Lyons B. L., Schwarz R. I. Ascorbate stimulation of PAT cells causes an increase in transcription rates and a decrease in degradation rates of procollagen mRNA. Nucleic Acids Res. 1984 Mar 12;12(5):2569–2579. doi: 10.1093/nar/12.5.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher J. J., McGuire R. F. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest. 1990 Nov;86(5):1641–1648. doi: 10.1172/JCI114886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M., Tsukamoto H. Stimulation of hepatic lipocyte collagen production by Kupffer cell-derived transforming growth factor beta: implication for a pathogenetic role in alcoholic liver fibrogenesis. Hepatology. 1990 Apr;11(4):599–605. doi: 10.1002/hep.1840110412. [DOI] [PubMed] [Google Scholar]
- Muir D., Varon S., Manthorpe M. An enzyme-linked immunosorbent assay for bromodeoxyuridine incorporation using fixed microcultures. Anal Biochem. 1990 Mar;185(2):377–382. doi: 10.1016/0003-2697(90)90310-6. [DOI] [PubMed] [Google Scholar]
- Okuyama R., Abo T., Seki S., Ohteki T., Sugiura K., Kusumi A., Kumagai K. Estrogen administration activates extrathymic T cell differentiation in the liver. J Exp Med. 1992 Mar 1;175(3):661–669. doi: 10.1084/jem.175.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paronetto F., Popper H. Chronic liver injury induced by immunologic reactions. Cirrhosis following immunization with heterologous sera. Am J Pathol. 1966 Dec;49(6):1087–1101. [PMC free article] [PubMed] [Google Scholar]
- Pinzani M., Gesualdo L., Sabbah G. M., Abboud H. E. Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. J Clin Invest. 1989 Dec;84(6):1786–1793. doi: 10.1172/JCI114363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzani M. Novel insights into the biology and physiology of the Ito cell. Pharmacol Ther. 1995 May;66(2):387–412. doi: 10.1016/0163-7258(94)00072-b. [DOI] [PubMed] [Google Scholar]
- Porter L. E., Elm M. S., Van Thiel D. H., Dugas M. C., Eagon P. K. Characterization and quantitation of human hepatic estrogen receptor. Gastroenterology. 1983 Apr;84(4):704–712. [PubMed] [Google Scholar]
- Ramadori G., Veit T., Schwögler S., Dienes H. P., Knittel T., Rieder H., Meyer zum Büschenfelde K. H. Expression of the gene of the alpha-smooth muscle-actin isoform in rat liver and in rat fat-storing (ITO) cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;59(6):349–357. doi: 10.1007/BF02899424. [DOI] [PubMed] [Google Scholar]
- Rockey D. C., Boyles J. K., Gabbiani G., Friedman S. L. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992 Apr;24(2):193–203. [PubMed] [Google Scholar]
- Rockey D. C., Chung J. J. Interferon gamma inhibits lipocyte activation and extracellular matrix mRNA expression during experimental liver injury: implications for treatment of hepatic fibrosis. J Investig Med. 1994 Dec;42(4):660–670. [PubMed] [Google Scholar]
- Rockey D. C., Maher J. J., Jarnagin W. R., Gabbiani G., Friedman S. L. Inhibition of rat hepatic lipocyte activation in culture by interferon-gamma. Hepatology. 1992 Sep;16(3):776–784. doi: 10.1002/hep.1840160325. [DOI] [PubMed] [Google Scholar]
- Rojkind M., Giambrone M. A., Biempica L. Collagen types in normal and cirrhotic liver. Gastroenterology. 1979 Apr;76(4):710–719. [PubMed] [Google Scholar]
- Rojkind M., Pérez-Tamayo R. Liver fibrosis. Int Rev Connect Tissue Res. 1983;10:333–393. doi: 10.1016/b978-0-12-363710-9.50012-5. [DOI] [PubMed] [Google Scholar]
- Rossini G. P., Baldini G. M., Villa E., Manenti F. Characterization of estrogen receptor from human liver. Gastroenterology. 1989 Apr;96(4):1102–1109. doi: 10.1016/0016-5085(89)91629-6. [DOI] [PubMed] [Google Scholar]
- Rubin E., Hutterer F., Popper H. Experimental hepatic fibrosis without hepatocellular regeneration. A kinetic study. Am J Pathol. 1968 Jan;52(1):111–120. [PMC free article] [PubMed] [Google Scholar]
- Seifert W. F., Bosma A., Brouwer A., Hendriks H. F., Roholl P. J., van Leeuwen R. E., van Thiel-de Ruiter G. C., Seifert-Bock I., Knook D. L. Vitamin A deficiency potentiates carbon tetrachloride-induced liver fibrosis in rats. Hepatology. 1994 Jan;19(1):193–201. [PubMed] [Google Scholar]
- Senoo H., Wake K. Suppression of experimental hepatic fibrosis by administration of vitamin A. Lab Invest. 1985 Feb;52(2):182–194. [PubMed] [Google Scholar]
- Shiba M., Shimizu I., Yasuda M., Ii K., Ito S. Expression of type I and type III collagens during the course of dimethylnitrosamine-induced hepatic fibrosis in rats. Liver. 1998 Jun;18(3):196–204. doi: 10.1111/j.1600-0676.1998.tb00150.x. [DOI] [PubMed] [Google Scholar]
- Tsipouras P., Myers J. C., Ramirez F., Prockop D. J. Restriction fragment length polymorphism associated with the pro alpha 2(I) gene of human type I procollagen. Application to a family with an autosomal dominant form of osteogenesis imperfecta. J Clin Invest. 1983 Oct;72(4):1262–1267. doi: 10.1172/JCI111082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa E., Baldini G. M., Pasquinelli C., Melegari M., Cariani E., Di Chirico G., Manenti F. Risk factors for hepatocellular carcinoma in Italy. Male sex, hepatitis B virus, non-A non-B infection, and alcohol. Cancer. 1988 Aug 1;62(3):611–615. doi: 10.1002/1097-0142(19880801)62:3<611::aid-cncr2820620328>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Zaman S. N., Melia W. M., Johnson R. D., Portmann B. C., Johnson P. J., Williams R. Risk factors in development of hepatocellular carcinoma in cirrhosis: prospective study of 613 patients. Lancet. 1985 Jun 15;1(8442):1357–1360. doi: 10.1016/s0140-6736(85)91785-4. [DOI] [PubMed] [Google Scholar]