Abstract
Background—Chronic dietary K+ loading increases the abundance of large conductance (210 pS) apical K+ channels in surface cells of rat distal colon, resulting in enhanced K+ secretion in this epithelium. However, the factors involved in the regulation of these K+ channels are at present unclear. Aims—To evaluate the effect of dietary K+ loading on intracellular pH and its relation to large conductance apical K+ channel activity in surface cells of rat distal colon. Methods/Results—As assessed by fluorescent imaging, intracellular pH was higher in K+ loaded animals (7.48 (0.09)) than in controls (7.07 (0.04); p<0.01) when surface cells were bathed in NaCl solution, and a similar difference in intracellular pH was observed when cells were bathed in Na2SO4 solution (7.67 (0.09) and 6.92 (0.05) respectively; p<0.001). Ethylisopropylamiloride (EIPA; an inhibitor of Na+-H+ exchange; 1 µM) decreased intracellular pH when surface cells from K+ loaded animals were bathed in either solution, although the decrease was greater when the solution contained NaCl (ΔpH 0.50 (0.03)) rather than Na2SO4 (ΔpH 0.18 (0.02); p<0.05). In contrast, EIPA had no effect in cells from control animals. As assessed by patch clamp recording techniques, the activity of large conductance K+ channels in excised inside-out membrane patches from distal colonic surface cells of K+ loaded animals increased twofold when the bath pH was raised from 7.40 to 7.60. As assessed by cell attached patches in distal colonic surface cells from K+ loaded animals, the addition of 1 µM EIPA decreased K+ channel activity by 50%, consistent with reversal of Na+-H+ exchange mediated intracellular alkalinisation. Conclusion—Intracellular alkalinisation stimulates pH sensitive large conductance apical K+ channels in rat distal colonic surface cells as part of the K+ secretory response to chronic dietary K+ loading. Intracellular alkalinisation seems to reflect an increase in EIPA sensitive Na+-H+ exchange, which may be a manifestation of the secondary hyperaldosteronism associated with this model of colonic K+ adaptation.
Keywords: colon; dietary potassium; pH; potassium channels
Full Text
The Full Text of this article is available as a PDF (162.4 KB).
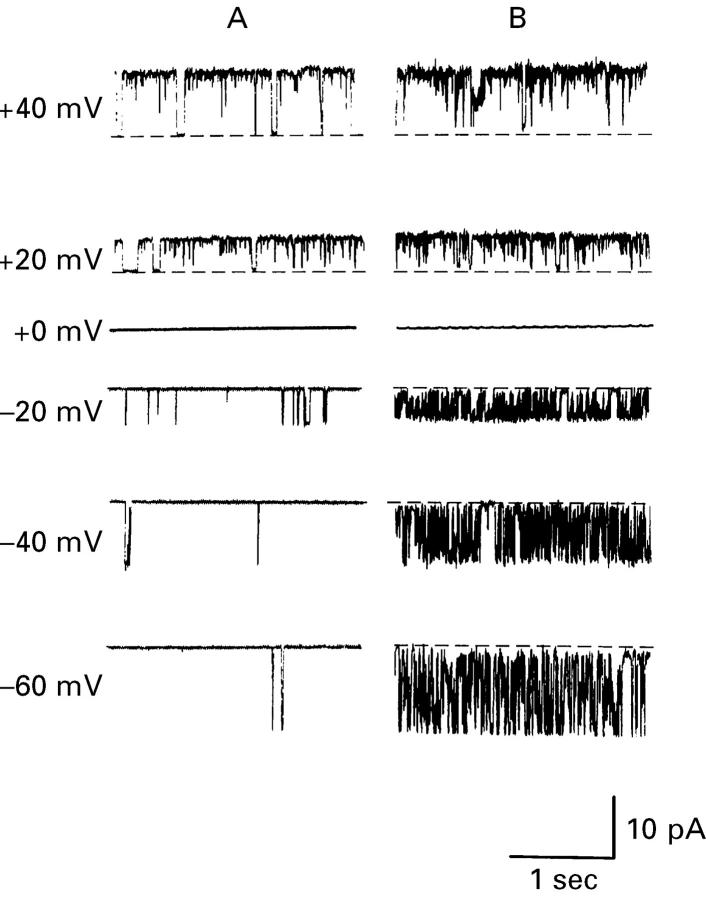
Figure 1 .
Effect of pH on large conductance K+ channels in isolated surface cells from rat distal colon. Typical recordings from an inside-out patch of membrane from a distal colonic surface cell isolated from K+ loaded animals at different Vcom values, referenced to the pipette interior (K2SO4 solution containing 32 nM Ca2+ in bath, K2SO4 solution containing 1.2 mM Ca2+ in the pipette). (A) and (B) Recordings at bath pH values of 7.40 and 7.60 respectively. The dashed lines indicate zero current levels, upward current deflections indicate K+ flow from the bath to the pipette, and downward current deflections indicate K+ flow from the pipette to the bath.
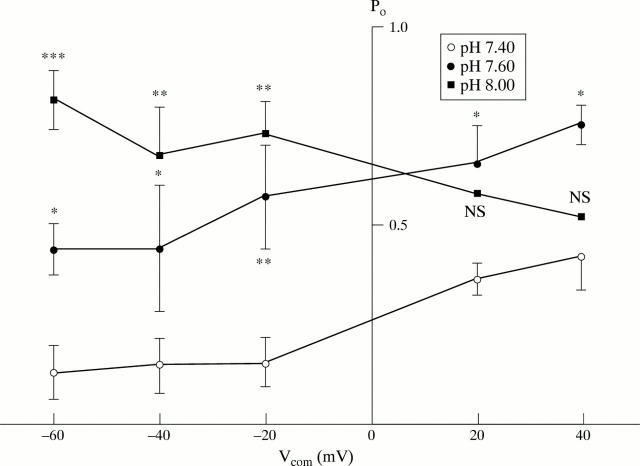
Figure 2 .
Effect of pH on large conductance K+ channel activity in isolated surface cells from rat distal colon. Po was determined over a range of Vcom values using inside-out patches from distal colonic surface cells isolated from K+ loaded animals (K2SO4 solution containing 32 nM Ca2+ in bath, K2SO4 solution containing 1.2 mM Ca2+ in the pipette). Data at pH 7.40, 7.60, and 8.00 were obtained from five to eight patches. *p<0.05, **p<0.01, and ***p<0.001 compared with value at pH 7.40. NS, not significant compared with value at 7.40.
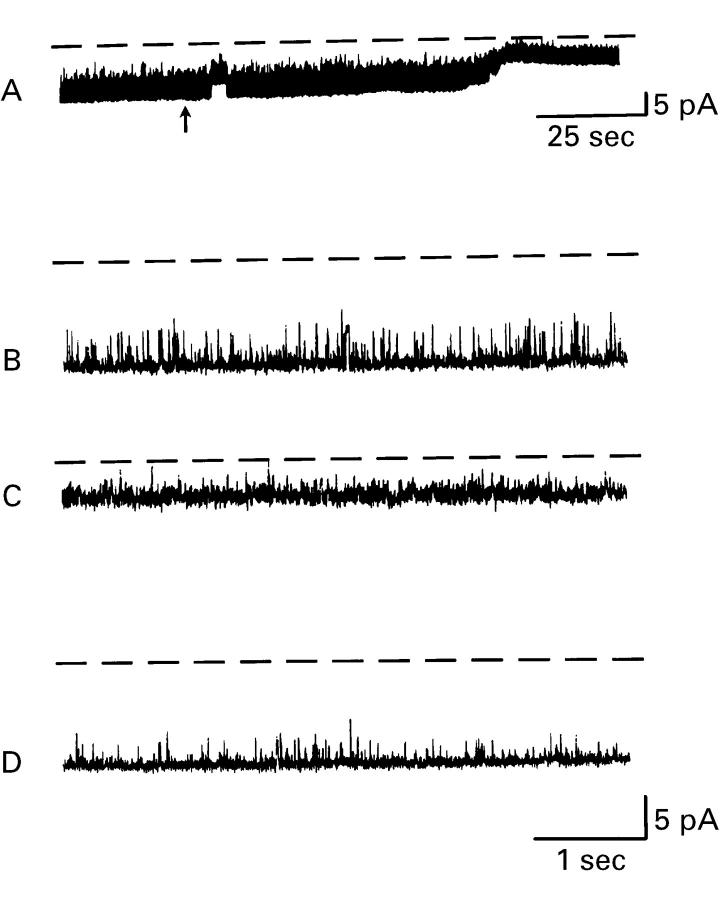
Figure 3 .
Effect of 1 µM ethylisopropylamiloride (EIPA) on large conductance K+ channels in isolated surface cells from rat distal colon. Typical recording from a cell attached patch of membrane from a distal colonic surface cell isolated from K+ loaded animal (Na2SO4 solution in bath, K2SO4 solution in the pipette; Vcom = 0 mV). (A) K+ channel activity on slow time base before and after the addition of 1 µM EIPA to the bath (denoted by arrow). Sections of (A) are presented on a faster time base to compare K+ channel activity before the addition of EIPA (B) and afterwards (C). (D) Restoration of channel activity after removal of EIPA. The dashed lines indicate zero current levels, and downward deflections indicate K+ flow from the pipette to the cell.
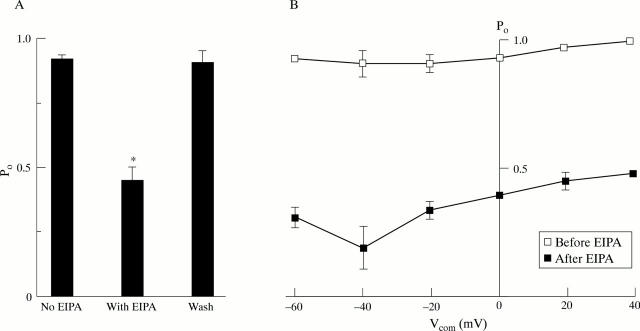
Figure 4 .
Effect of 1 µM ethylisopropylamiloride (EIPA) on large conductance K+ channel activity in isolated surface cells from rat distal colon. (A) Summary of results from five cell attached patches of membrane from distal colonic surface cells isolated from K+ loaded animals (Na2SO4 solution in bath, K2SO4 solution in the pipette; Vcom = 0 mV). *p<0.001 compared with the value before the addition of EIPA. (B) Uniform effect of EIPA on K+ channel activity at holding voltages between −60 and 40 mV (n = 3 patches). Error bars not shown were smaller than the symbols. Differences between the values before and after the addition of EIPA were highly significant (p<0.001) at each holding voltage.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butterfield I., Warhurst G., Jones M. N., Sandle G. I. Characterization of apical potassium channels induced in rat distal colon during potassium adaptation. J Physiol. 1997 Jun 15;501(Pt 3):537–547. doi: 10.1111/j.1469-7793.1997.537bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Castillo J. R., Rajendran V. M., Binder H. J. Apical membrane localization of ouabain-sensitive K(+)-activated ATPase activities in rat distal colon. Am J Physiol. 1991 Dec;261(6 Pt 1):G1005–G1011. doi: 10.1152/ajpgi.1991.261.6.G1005. [DOI] [PubMed] [Google Scholar]
- Duffey M. E., Devor D. C. Intracellular pH and membrane potassium conductance in rabbit distal colon. Am J Physiol. 1990 Feb;258(2 Pt 1):C336–C343. doi: 10.1152/ajpcell.1990.258.2.C336. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Foster E. S., Sandle G. I., Hayslett J. P., Binder H. J. Dietary potassium modulates active potassium absorption and secretion in rat distal colon. Am J Physiol. 1986 Nov;251(5 Pt 1):G619–G626. doi: 10.1152/ajpgi.1986.251.5.G619. [DOI] [PubMed] [Google Scholar]
- Foster E. S., Zimmerman T. W., Hayslett J. P., Binder H. J. Corticosteroid alteration of active electrolyte transport in rat distal colon. Am J Physiol. 1983 Nov;245(5 Pt 1):G668–G675. doi: 10.1152/ajpgi.1983.245.5.G668. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hawker P. C., Mashiter K. E., Turnberg L. A. Mechanisms of transport of Na, Cl, and K in the human colon. Gastroenterology. 1978 Jun;74(6):1241–1247. [PubMed] [Google Scholar]
- Kashgarian M., Taylor C. R., Binder H. J., Hayslett J. P. Amplification of cell membrane surface in potassium adaptation. Lab Invest. 1980 Jun;42(6):581–588. [PubMed] [Google Scholar]
- Martin R. S., Jones W. J., Hayslett J. P. Animal model to study the effect of adrenal hormones on epithelial function. Kidney Int. 1983 Sep;24(3):386–391. doi: 10.1038/ki.1983.171. [DOI] [PubMed] [Google Scholar]
- Muto S., Sansom S., Giebisch G. Effects of a high potassium diet on electrical properties of cortical collecting ducts from adrenalectomized rabbits. J Clin Invest. 1988 Feb;81(2):376–380. doi: 10.1172/JCI113329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberleithner H., Kersting U., Hunter M. Cytoplasmic pH determines K+ conductance in fused renal epithelial cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8345–8349. doi: 10.1073/pnas.85.21.8345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberleithner H., Weigt M., Westphale H. J., Wang W. Aldosterone activates Na+/H+ exchange and raises cytoplasmic pH in target cells of the amphibian kidney. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1464–1468. doi: 10.1073/pnas.84.5.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran V. M., Binder H. J. Differential modulation of Na-HCO3 cotransport and Na-H exchange by pH in basolateral membrane vesicles of rat distal colon. J Biol Chem. 1994 Jan 7;269(1):156–160. [PubMed] [Google Scholar]
- Rajendran V. M., Geibel J., Binder H. J. Chloride-dependent Na-H exchange. A novel mechanism of sodium transport in colonic crypts. J Biol Chem. 1995 May 12;270(19):11051–11054. doi: 10.1074/jbc.270.19.11051. [DOI] [PubMed] [Google Scholar]
- Sandle G. I., Foster E. S., Lewis S. A., Binder H. J., Hayslett J. P. The electrical basis for enhanced potassium secretion in rat distal colon during dietary potassium loading. Pflugers Arch. 1985 Apr;403(4):433–439. doi: 10.1007/BF00589258. [DOI] [PubMed] [Google Scholar]
- Sandle G. I., Gaiger E., Tapster S., Goodship T. H. Enhanced rectal potassium secretion in chronic renal insufficiency: evidence for large intestinal potassium adaptation in man. Clin Sci (Lond) 1986 Oct;71(4):393–401. doi: 10.1042/cs0710393. [DOI] [PubMed] [Google Scholar]
- Sandle G. I., Gaiger E., Tapster S., Goodship T. H. Evidence for large intestinal control of potassium homoeostasis in uraemic patients undergoing long-term dialysis. Clin Sci (Lond) 1987 Sep;73(3):247–252. doi: 10.1042/cs0730247. [DOI] [PubMed] [Google Scholar]
- Stieger B., Marxer A., Hauri H. P. Isolation of brush-border membranes from rat and rabbit colonocytes: is alkaline phosphatase a marker enzyme? J Membr Biol. 1986;91(1):19–31. doi: 10.1007/BF01870211. [DOI] [PubMed] [Google Scholar]
- Sweiry J. H., Binder H. J. Active potassium absorption in rat distal colon. J Physiol. 1990 Apr;423:155–170. doi: 10.1113/jphysiol.1990.sp018016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills N. K., Alles W. P., Sandle G. I., Binder H. J. Apical membrane properties and amiloride binding kinetics of the human descending colon. Am J Physiol. 1984 Dec;247(6 Pt 1):G749–G757. doi: 10.1152/ajpgi.1984.247.6.G749. [DOI] [PubMed] [Google Scholar]