Abstract
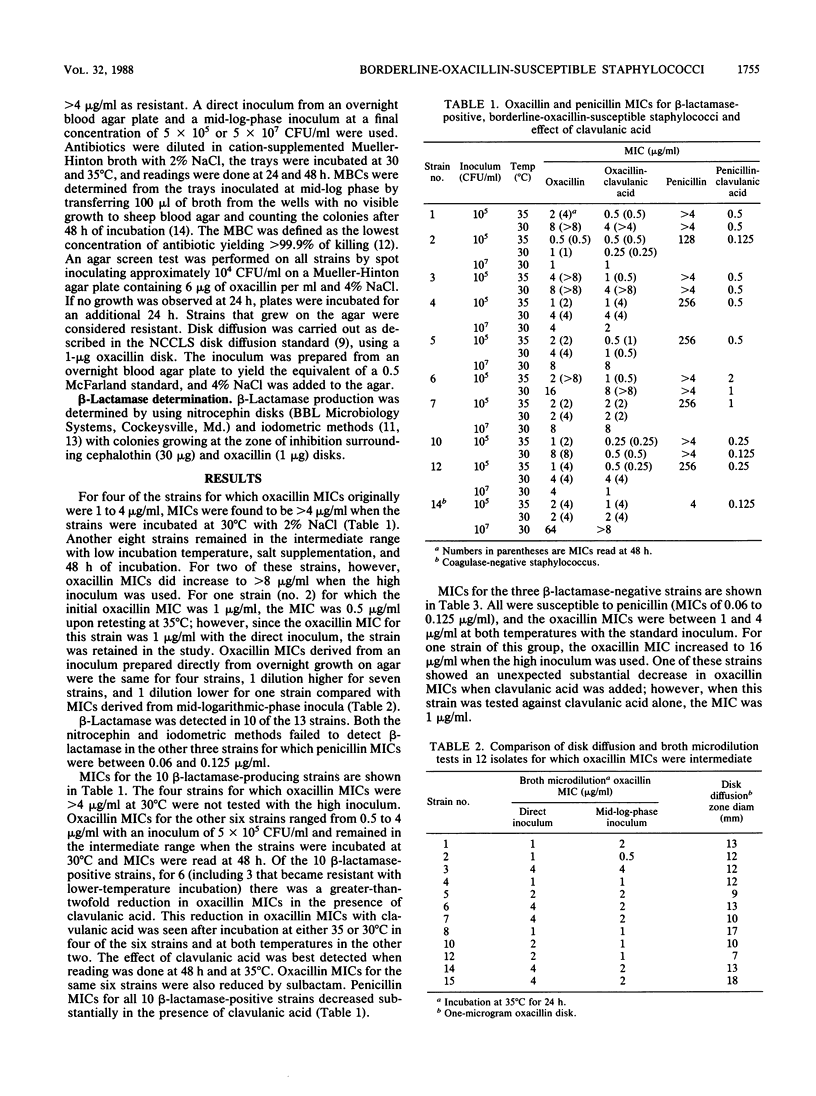
A group of staphylococcal isolates for which oxacillin MICs were intermediate (1 to 4 micrograms/ml) were studied to establish the role of beta-lactamase in this phenomenon. MICs and MBCs of oxacillin and penicillin with and without clavulanic acid or sulbactam (4 or 16 micrograms/ml, respectively) were determined for 11 Staphylococcus aureus and 2 coagulase-negative Staphylococcus isolates for which oxacillin MICs were 1 to 4 micrograms/ml. The susceptibility studies were done with incubation at 35 and 30 degrees C, and the MICs were read at 24 and 48 h. Of the 13 isolates, 4 became resistant when longer incubation or 30 degrees C incubation was used, and the MICs for 9 remained in the intermediate range. Only three of these strains were susceptible to penicillin, and beta-lactamase was not detected. For 6 of 10 beta-lactamase-positive strains, there was a greater-than-twofold-dilution reduction in oxacillin MICs with the addition of clavulanic acid or sulbactam. Of the four strains that became resistant with incubation at the lower temperature, a clavulanic acid effect was observed in three but only at 35 degrees C. The oxacillin MIC for one of the beta-lactamase-negative strains was also reduced with clavulanic acid; however, this strain was inhibited by 1 microgram of clavulanic acid per ml alone. Bactericidal activity was observed with two or four times the oxacillin MIC in eight strains tested at both temperatures, and the combination with clavulanic acid was bactericidal at higher than four times the MIC in five of the strains at 30 degrees C. Our results suggest that oxacillin intermediate MICs for staphylococcal isolates are due not only to beta-lactamase hyperproduction but also some other unidentified factor. The reduction in oxacillin MIC observed when clavulanic acid was added to one strain was probably due to the intrinsic inhibitory activity of clavulanic acid.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Forbes B. A., McClatchey K. D., Schaberg D. R. Subinhibitory concentrations of imipenem induce increased resistance to methicillin and imipenem in vitro in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1984 Apr;25(4):491–493. doi: 10.1128/aac.25.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Smith S. A., Bonner D. P. Penicillin-binding proteins in a Staphylococcus aureus strain resistant to specific beta-lactam antibiotics. Antimicrob Agents Chemother. 1982 Jul;22(1):172–175. doi: 10.1128/aac.22.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984 May;158(2):513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimek J. J., Marsik F. J., Bartlett R. C., Weir B., Shea P., Quintiliani R. Clinical, epidemiologic and bacteriologic observations of an outbreak of methicillin-resistant Staphylococcus aureus at a large community hospital. Am J Med. 1976 Sep;61(3):340–345. doi: 10.1016/0002-9343(76)90370-3. [DOI] [PubMed] [Google Scholar]
- McDougal L. K., Thornsberry C. New recommendations for disk diffusion antimicrobial susceptibility tests for methicillin-resistant (heteroresistant) staphylococci. J Clin Microbiol. 1984 Apr;19(4):482–488. doi: 10.1128/jcm.19.4.482-488.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal L. K., Thornsberry C. The role of beta-lactamase in staphylococcal resistance to penicillinase-resistant penicillins and cephalosporins. J Clin Microbiol. 1986 May;23(5):832–839. doi: 10.1128/jcm.23.5.832-839.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T., Davis H. T., Chapman S. W. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980 Nov;18(5):699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J. E., Neumann A. M. A rapid slide test for penicillinase. Am J Clin Pathol. 1978 Mar;69(3):351–354. doi: 10.1093/ajcp/69.1.351. [DOI] [PubMed] [Google Scholar]
- Shanholtzer C. J., Peterson L. R., Mohn M. L., Moody J. A., Gerding D. N. MBCs for Staphylococcus aureus as determined by macrodilution and microdilution techniques. Antimicrob Agents Chemother. 1984 Aug;26(2):214–219. doi: 10.1128/aac.26.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrell T. C., Packham D. R., Shanker S., Foldes M., Munro R. Vancomycin therapy for methicillin-resistant Staphylococcus aureus. Ann Intern Med. 1982 Sep;97(3):344–350. doi: 10.7326/0003-4819-97-3-344. [DOI] [PubMed] [Google Scholar]
- Thornsberry C., McDougal L. K. Successful use of broth microdilution in susceptibility tests for methicillin-resistant (heteroresistant) staphylococci. J Clin Microbiol. 1983 Nov;18(5):1084–1091. doi: 10.1128/jcm.18.5.1084-1091.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



