Abstract
BACKGROUND/AIMS—We aimed to evaluate the role of fat and cholecystokinin (CCK) in the pathophysiology of functional dyspepsia (FD) by investigating symptoms and plasma CCK levels following increasing doses of duodenal lipid during gastric distension, and the effect of CCK-A receptor blockade. SUBJECTS/METHODS—In study A, six FD patients were studied on three occasions during duodenal infusion of saline or lipid (1.1 (L-1) or 2 kcal/min (L-2)) and proximal gastric distensions. Six healthy subjects were also studied as controls during L-2 only. In study B, the effect of the CCK-A antagonist dexloxiglumide (5 mg/kg/h) on L-2 induced symptoms was studied in 12 FD patients. Changes in gastric volume at minimal distending pressure and plasma CCK (study A) were assessed, gastric distensions were performed using a barostat, and dyspeptic symptoms were monitored. RESULTS—Lipid increased gastric volume compared with saline (ΔV (ml): saline 15 (20), L-1 122 (42), L-2 114 (28)) in patients and even more so in controls (221 (37); p<0.05). During distensions, symptoms were greater during L-2 than during saline or L-1, and greater in patients than in controls, while gastric compliance was smaller in patients than in controls (p<0.05). Lipid increased plasma CCK levels in patients and controls (p>0.05). Dexloxiglumide abolished the increase in gastric volume (ΔV (ml): dexloxiglumide 17 (9), placebo 186 (49)) and dyspeptic symptoms (sum of scores: dexloxiglumide 24 (7), placebo 44 (19)) during duodenal lipid infusion. Dexloxiglumide also reduced gastric compliance (ml/mm Hg: dexloxiglumide 51 (7), placebo 72 (11)) and symptoms (sum of scores: dexloxiglumide 101 (17), placebo 154 (21)) during gastric distension. CONCLUSION—CCK-A receptors are involved in the generation of dyspeptic symptoms by duodenal lipid during gastric distension. Keywords: functional dyspepsia; duodenal lipid; cholecystokinin; dyspeptic symptoms
Full Text
The Full Text of this article is available as a PDF (173.4 KB).
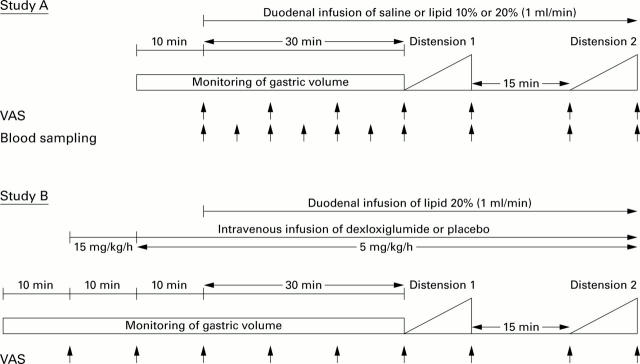
Figure 1 .
Experimental protocols for study A (dose-response study) and study B (cholecystokinin (CCK)-A receptor blockade). To determine the relationship between the lipid dose administered and severity of dyspeptic symptoms, six patients were studied on three occasions each, receiving duodenal infusion of 0.9% saline, or 10% or 20% lipid. Six healthy subjects were studied after infusion of 20% lipid only. During infusions, changes in gastric volume were assessed and the proximal stomach was distended in steps of 1 mm Hg/min while symptoms were monitored and plasma CCK levels determined. In study B, 12 patients were studied twice to investigate the effect of CCK-A receptor blockade with dexloxiglumide on the symptomatic and gastric volume responses evoked by duodenal infusion of 20% lipid and gastric distension. VAS, visual analogue scale.
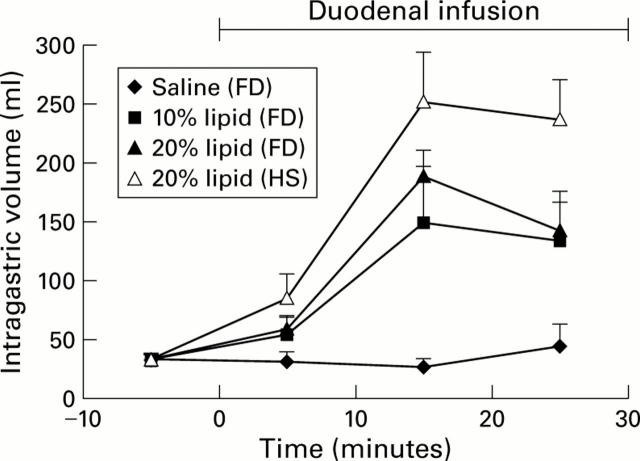
Figure 2 .
Gastric volume responses to duodenal infusion of isotonic saline, or 10% or 20% lipid in patients with functional dyspepsia (FD) and to infusion of 20% lipid in healthy subjects (HS) (n = 6).
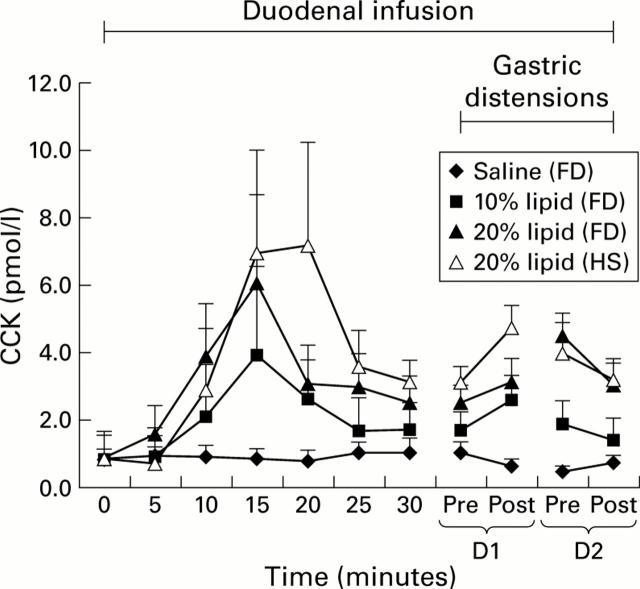
Figure 3 .
Plasma cholecystokinin (CCK) levels (baseline levels normalised) obtained in response to duodenal infusion of isotonic saline, or 10% or 20% lipid (n=6) in patients with functional dyspepsia (FD) and healthy subjects (HS) before and during gastric distensions. The value at t=20 minutes in healthy subjects is due to one outlier. D1, distension 1; D2, distension 2.
Figure 4 .
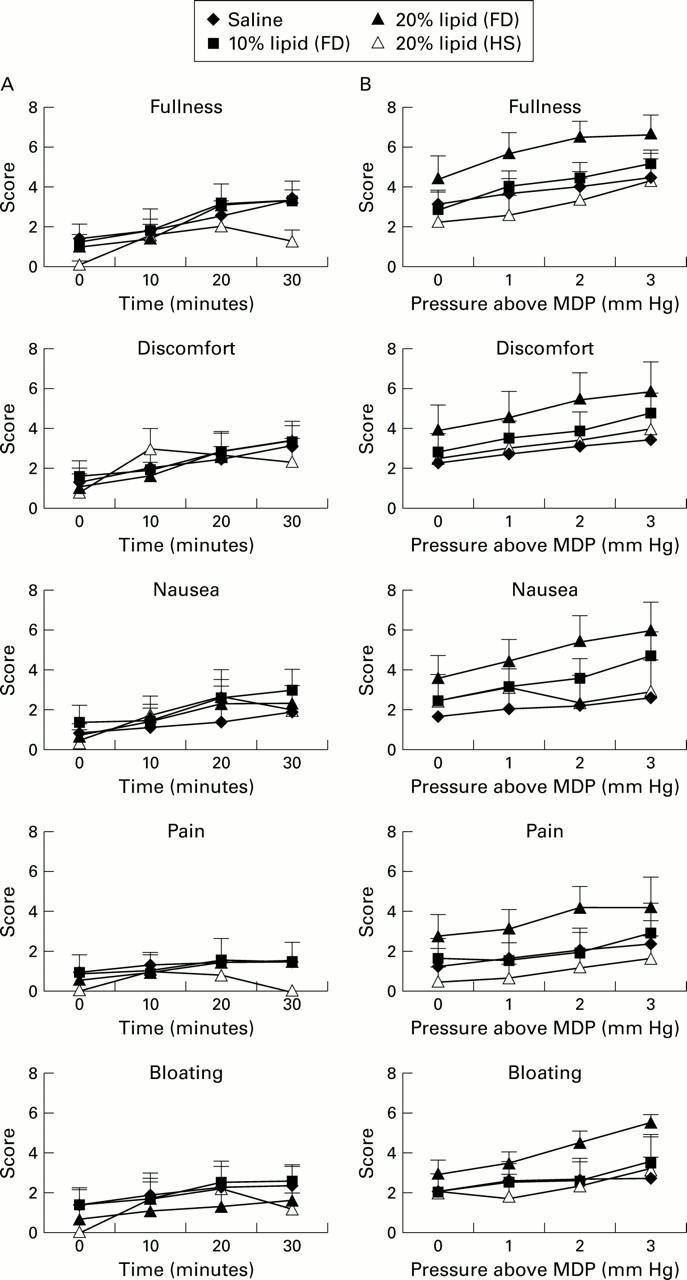
Scores for dyspeptic symptoms during duodenal infusion of isotonic saline, or 10% or 20% lipid in patients with functional dyspepsia (FD) and 20% lipid in healthy subjects (HS) (A) and when infusions were combined with gastric distension (B).
Figure 5 .
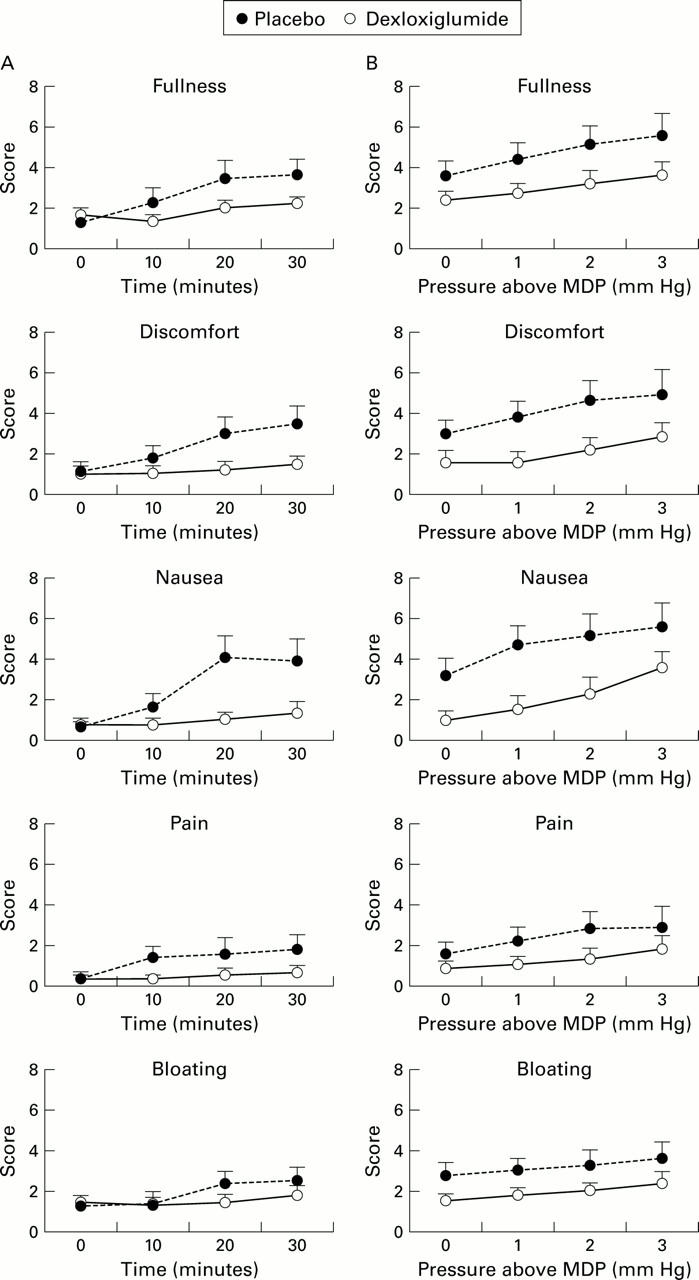
Scores for dyspeptic symptoms during duodenal infusion of 20% lipid given with intravenous placebo or dexloxiglumide in patients with functional dyspepsia (A) and when infusions were combined with gastric distension (B).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. L., Davis C. J., Bingham S., Davidson H. I., Hawthorn J., Maskell L. The abdominal visceral innervation and the emetic reflex: pathways, pharmacology, and plasticity. Can J Physiol Pharmacol. 1990 Feb;68(2):325–345. doi: 10.1139/y90-047. [DOI] [PubMed] [Google Scholar]
- Azpiroz F., Malagelada J. R. Intestinal control of gastric tone. Am J Physiol. 1985 Oct;249(4 Pt 1):G501–G509. doi: 10.1152/ajpgi.1985.249.4.G501. [DOI] [PubMed] [Google Scholar]
- Barbera R., Feinle C., Read N. W. Abnormal sensitivity to duodenal lipid infusion in patients with functional dyspepsia. Eur J Gastroenterol Hepatol. 1995 Nov;7(11):1051–1057. doi: 10.1097/00042737-199511000-00007. [DOI] [PubMed] [Google Scholar]
- Barbera R., Feinle C., Read N. W. Nutrient-specific modulation of gastric mechanosensitivity in patients with functional dyspepsia. Dig Dis Sci. 1995 Aug;40(8):1636–1641. doi: 10.1007/BF02212683. [DOI] [PubMed] [Google Scholar]
- Bradette M., Pare P., Douville P., Morin A. Visceral perception in health and functional dyspepsia. Crossover study of gastric distension with placebo and domperidone. Dig Dis Sci. 1991 Jan;36(1):52–58. doi: 10.1007/BF01300087. [DOI] [PubMed] [Google Scholar]
- Cann P. A., Rovati L. C., Smart H. L., Spiller R. C., Whorwell P. J. Loxiglumide, a CCK-A antagonist, in irritable bowel syndrome. A pilot multicenter clinical study. Ann N Y Acad Sci. 1994 Mar 23;713:449–450. doi: 10.1111/j.1749-6632.1994.tb44123.x. [DOI] [PubMed] [Google Scholar]
- Chua A. S., Bekkering M., Rovati L. C., Keeling P. W. Clinical efficacy and prokinetic effect of the CCK-A antagonist loxiglumide in nonulcer dyspepsia. Ann N Y Acad Sci. 1994 Mar 23;713:451–453. doi: 10.1111/j.1749-6632.1994.tb44124.x. [DOI] [PubMed] [Google Scholar]
- Chua A. S., Dinan T. G., Rovati L. C., Keeling P. W. Cholecystokinin hyperresponsiveness in dysmotility-type nonulcer dyspepsia. Ann N Y Acad Sci. 1994 Mar 23;713:298–299. doi: 10.1111/j.1749-6632.1994.tb44077.x. [DOI] [PubMed] [Google Scholar]
- D'Amato M., Rovati L. C. Cholecystokinin-A receptor antagonists: therapies for gastrointestinal disorders. Expert Opin Investig Drugs. 1997 Jul;6(7):819–836. doi: 10.1517/13543784.6.7.819. [DOI] [PubMed] [Google Scholar]
- Feinle C., D'Amato M., Read N. W. Cholecystokinin-A receptors modulate gastric sensory and motor responses to gastric distension and duodenal lipid. Gastroenterology. 1996 May;110(5):1379–1385. doi: 10.1053/gast.1996.v110.pm8613041. [DOI] [PubMed] [Google Scholar]
- Feinle C., Grundy D., Read N. W. Effects of duodenal nutrients on sensory and motor responses of the human stomach to distension. Am J Physiol. 1997 Sep;273(3 Pt 1):G721–G726. doi: 10.1152/ajpgi.1997.273.3.G721. [DOI] [PubMed] [Google Scholar]
- Gilja O. H., Hausken T., Wilhelmsen I., Berstad A. Impaired accommodation of proximal stomach to a meal in functional dyspepsia. Dig Dis Sci. 1996 Apr;41(4):689–696. doi: 10.1007/BF02213124. [DOI] [PubMed] [Google Scholar]
- Hopman W. P., Jansen J. B., Lamers C. B. Comparative study of the effects of equal amounts of fat, protein, and starch on plasma cholecystokinin in man. Scand J Gastroenterol. 1985 Sep;20(7):843–847. doi: 10.3109/00365528509088832. [DOI] [PubMed] [Google Scholar]
- Koop I., Ruppert-Seipp G., Koop H., Schafmayer A., Arnold R. Cholecystokinin release by gastric distension--an atropine-sensitive mechanism. Digestion. 1990;46(4):220–227. doi: 10.1159/000200349. [DOI] [PubMed] [Google Scholar]
- Kreiss C., Schwizer W., Erlacher U., Borovicka J., Löchner-Kuery C., Müller R., Jansen J. B., Fried M. Role of antrum in regulation of pancreaticobiliary secretion in humans. Am J Physiol. 1996 May;270(5 Pt 1):G844–G851. doi: 10.1152/ajpgi.1996.270.5.G844. [DOI] [PubMed] [Google Scholar]
- Kvietys P. R., Specian R. D., Grisham M. B., Tso P. Jejunal mucosal injury and restitution: role of hydrolytic products of food digestion. Am J Physiol. 1991 Sep;261(3 Pt 1):G384–G391. doi: 10.1152/ajpgi.1991.261.3.G384. [DOI] [PubMed] [Google Scholar]
- Lieverse R. J., Jansen J. B., Lamers C. B. Cholecystokinin and satiation. Neth J Med. 1993 Apr;42(3-4):146–152. [PubMed] [Google Scholar]
- Lémann M., Dederding J. P., Flourié B., Franchisseur C., Rambaud J. C., Jian R. Abnormal perception of visceral pain in response to gastric distension in chronic idiopathic dyspepsia. The irritable stomach syndrome. Dig Dis Sci. 1991 Sep;36(9):1249–1254. doi: 10.1007/BF01307517. [DOI] [PubMed] [Google Scholar]
- Malagelada J. R. The quest for a physiological answer to dyspepsia. Gastroenterology. 1998 Dec;115(6):1586–1588. doi: 10.1016/s0016-5085(98)70041-1. [DOI] [PubMed] [Google Scholar]
- Mearin F., Cucala M., Azpiroz F., Malagelada J. R. The origin of symptoms on the brain-gut axis in functional dyspepsia. Gastroenterology. 1991 Oct;101(4):999–1006. doi: 10.1016/0016-5085(91)90726-2. [DOI] [PubMed] [Google Scholar]
- Mesquita M. A., Thompson D. G., Troncon L. E., D'Amato M., Rovati L. C., Barlow J. Effect of cholecystokinin-A receptor blockade on lipid-induced gastric relaxation in humans. Am J Physiol. 1997 Jul;273(1 Pt 1):G118–G123. doi: 10.1152/ajpgi.1997.273.1.G118. [DOI] [PubMed] [Google Scholar]
- Miaskiewicz S. L., Stricker E. M., Verbalis J. G. Neurohypophyseal secretion in response to cholecystokinin but not meal-induced gastric distention in humans. J Clin Endocrinol Metab. 1989 Apr;68(4):837–843. doi: 10.1210/jcem-68-4-837. [DOI] [PubMed] [Google Scholar]
- Naliboff B. D., Munakata J., Fullerton S., Gracely R. H., Kodner A., Harraf F., Mayer E. A. Evidence for two distinct perceptual alterations in irritable bowel syndrome. Gut. 1997 Oct;41(4):505–512. doi: 10.1136/gut.41.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederau C., Heintges T., Rovati L., Strohmeyer G. Effects of loxiglumide on gallbladder emptying in healthy volunteers. Gastroenterology. 1989 Nov;97(5):1331–1336. doi: 10.1016/0016-5085(89)91709-5. [DOI] [PubMed] [Google Scholar]
- Riepl R. L., Fiedler F., Ernstberger M., Teufel J., Lehnert P. Effect of intraduodenal taurodeoxycholate and L-phenylalanine on pancreatic secretion and on gastroenteropancreatic peptide release in man. Eur J Med Res. 1996 Sep 20;1(11):499–505. [PubMed] [Google Scholar]
- Sepple C. P., Read N. W. Gastrointestinal correlates of the development of hunger in man. Appetite. 1989 Dec;13(3):183–191. doi: 10.1016/0195-6663(89)90011-1. [DOI] [PubMed] [Google Scholar]
- Sternini C., Wong H., Pham T., De Giorgio R., Miller L. J., Kuntz S. M., Reeve J. R., Walsh J. H., Raybould H. E. Expression of cholecystokinin A receptors in neurons innervating the rat stomach and intestine. Gastroenterology. 1999 Nov;117(5):1136–1146. doi: 10.1016/s0016-5085(99)70399-9. [DOI] [PubMed] [Google Scholar]
- Taggart D., Billington B. P. Fatty foods and dyspersia. Lancet. 1966 Aug 27;2(7461):465–466. [PubMed] [Google Scholar]
- Troncon L. E., Bennett R. J., Ahluwalia N. K., Thompson D. G. Abnormal intragastric distribution of food during gastric emptying in functional dyspepsia patients. Gut. 1994 Mar;35(3):327–332. doi: 10.1136/gut.35.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez O. R., Henninger K., Fowler M., Tso P., Crissinger K. D. Oleic acid-induced mucosal injury in developing piglet intestine. Am J Physiol. 1993 Mar;264(3 Pt 1):G576–G582. doi: 10.1152/ajpgi.1993.264.3.G576. [DOI] [PubMed] [Google Scholar]