Abstract
Coeliac disease (CD) is caused by a CD4 T helper cell type 1 (Th1) response in the small intestinal mucosa to dietary gluten. As the major Th1 inducing cytokine, interleukin 12, is undetectable in CD gut mucosa, the mechanism by which Th1 effector cells are generated remains unknown. Interferon (IFN) α, a cytokine capable of promoting IFN-γ synthesis, has been implicated in the development of Th1 mediated immune diseases. Here we report a case of CD-like enteropathy in a patient receiving IFN-α for chronic myeloid leukaemia. Morphological assessment of duodenal biopsies taken from the patient showed total villous atrophy, crypt cell hyperplasia, and a high number of CD3+ intraepithelial lymphocytes. Both antigliadin antibodies and antiendomysial antibodies were positive. RNA analysis revealed pronounced expression of IFN-γ. Withdrawal of gluten from the diet resulted in a patchy improvement in intestinal morphology, normalisation of laboratory parameters, and resolution of clinical symptoms. By western blot analysis, IFN-α protein was seen in the duodenal mucosa from untreated CD patients but not in controls. This was associated with marked expression of IFN-γ protein in CD mucosa. Collectively, these results suggest a role for IFN-α in promoting Th1 responses to gluten. Keywords: coeliac disease; interferon; small intestine; T helper cell response
Full Text
The Full Text of this article is available as a PDF (158.7 KB).
Figure 1 .
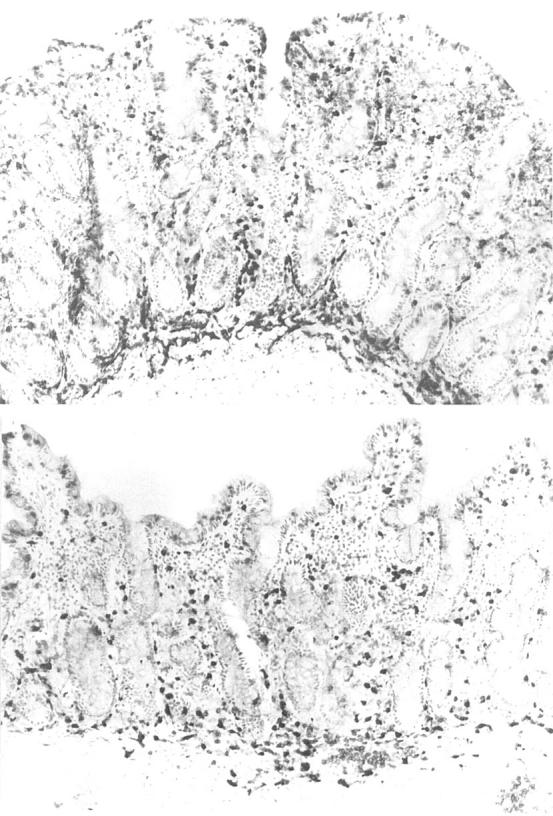
Frozen sections of duodenal biopsies taken from the patient receiving interferon α therapy before (top) and after (bottom) commencing a gluten free diet. Before the gluten free diet, the mucosa is flat, there is a dense infiltrate of intraepithelial lymphocytes (IEL), and there are a large number of non-specific inflammatory cells, as shown by the strongly positive endogenous peroxidase cells in the lamina propria. After four months of a gluten free diet, there are short villi, fewer IELs, and non-specific inflammatory cells are also markedly reduced. Immunoperoxidase with anti-CD3; original magnification ×120.
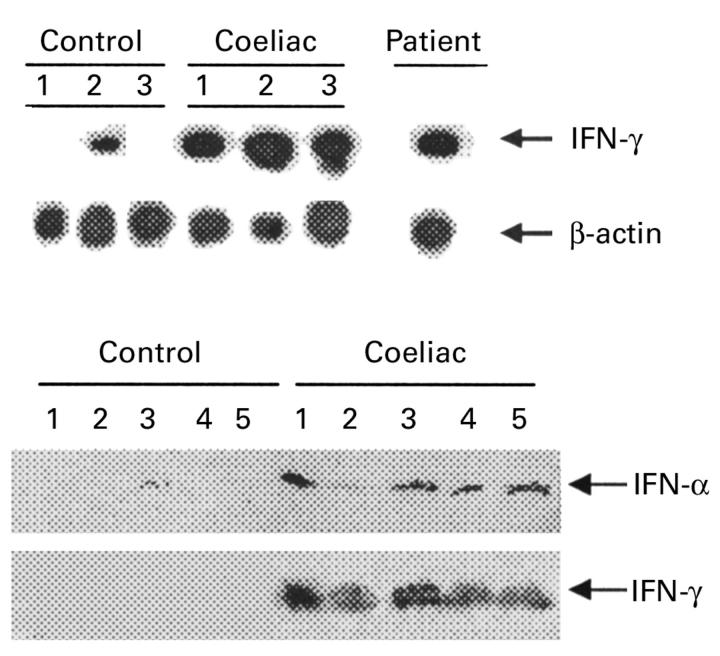
Figure 2 .
(Top) Southern blot analysis of transcripts for interferon (IFN) γ and β-actin in duodenal mucosal tissue homogenates from three normal controls, three untreated patients with coeliac disease (CD), and the patient with chronic myeloid leukaemia receiving IFN-α treatment before commencing a gluten free diet. A total of 3 or 1.5 µl of cDNA was amplified using specific primers for IFN-γ (23 cycles) or β-actin (19 cycles), respectively. RT-PCR products were separated on agarose gel, blotted, and hybridised with specific probes for IFN-γ or β-actin. (Bottom) Western blot analysis of IFN-α and IFN-γ protein expression in mucosal samples taken from the distal duodenum of five normal controls and five patients with untreated CD. IFN-α protein was detected in mucosal samples of all CD patients and in one (lane 3) normal control. After stripping, the blot was incubated with an anti-IFN-γ antibody. A protein of approximately 19 kDa was detected only in mucosal samples of CD patients. One of two representative experiments is shown.