Abstract
BACKGROUND/AIM—Inactivation of the p16INK4A (p16) tumour suppressor gene by promoter region hypermethylation has been demonstrated not only in many types of tumours, including hepatocellular carcinoma (HCC), but also in early preneoplastic lesions in the lung, colon, oesophagus, and pancreas. The aim of this study was to examine the methylation status of the p16 promoter in pre- and/or non-neoplastic liver diseases. PATIENTS/SUBJECTS/METHODS—The methylation status of p16 was evaluated in 22 HCC, 17 cirrhosis, 17 chronic hepatitis, nine primary biliary cirrhosis (PBC), eight autoimmune hepatitis, seven drug induced liver disease, six fatty liver, and three normal liver tissues using methylation specific polymerase chain reaction (MSP). p16 protein expression was also examined by immunohistochemical staining. RESULTS—Methylation of the p16 promoter was detected in HCC (72.7%, 16/22) and also in cirrhosis (29.4%, 5/17) and chronic hepatitis (23.5%, 4/17), all of which were positive for hepatitis B or C virus infections. Methylation was not detected in any of the other samples. All methylation positive HCC, cirrhosis, and chronic hepatitis samples showed loss of p16 expression, and a significant correlation was found between methylation and loss of expression. Analysis of serial samples from individual patients with methylation positive HCC revealed that loss of p16 expression with promoter methylation occurred in 18 of 20 patients at the stage of chronic hepatitis without clinically detectable carcinoma. CONCLUSIONS—Our results suggest that methylation of the p16 promoter and the resulting loss of p16 protein expression are early events in a subset of hepatocarcinogenesis and that their detection is useful in the follow up of patients with a high risk of developing HCC, such as those with hepatitis B or C viral infections. Keywords: hypermethylation; p16; hepatocarcinogenesis; preneoplastic diseases; hepatitis virus infection; methylation specific PCR
Full Text
The Full Text of this article is available as a PDF (250.6 KB).
Figure 1 .
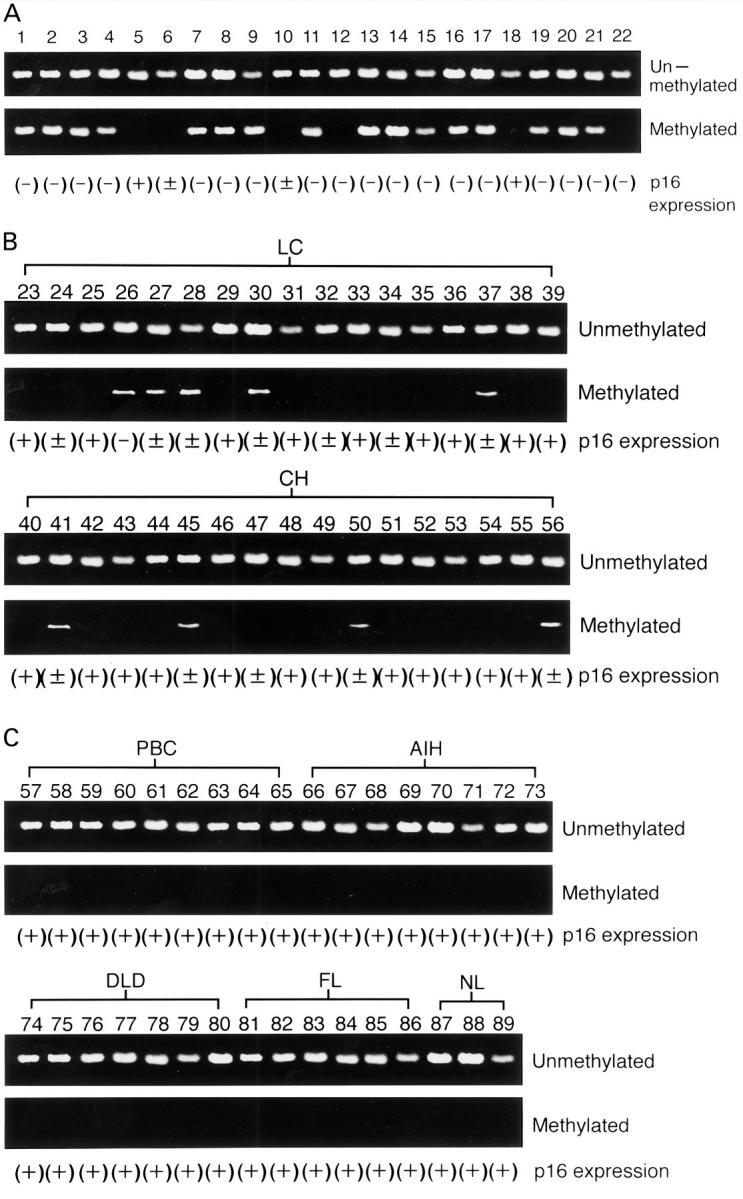
Methylation specific polymerase chain reaction (PCR) analysis and expression of p16 gene. (A) Hepatocellular carcinoma (HCC) samples. (B) Liver cirrhosis (LC) and chronic hepatitis (CH) samples. (C) Primary biliary cirrhosis (PBC), autoimmune hepatitis (AIH), drug induced liver disease (DLD), fatty liver (FL), and normal liver (NL) samples. Unmethylated and methylated indicate methylation specific PCR analysis specific for unmethylated and methylated promoter of p16, respectively. (+), normal expression of p16; (±), partial loss of p16 expression; (−), complete loss of p16 expression.
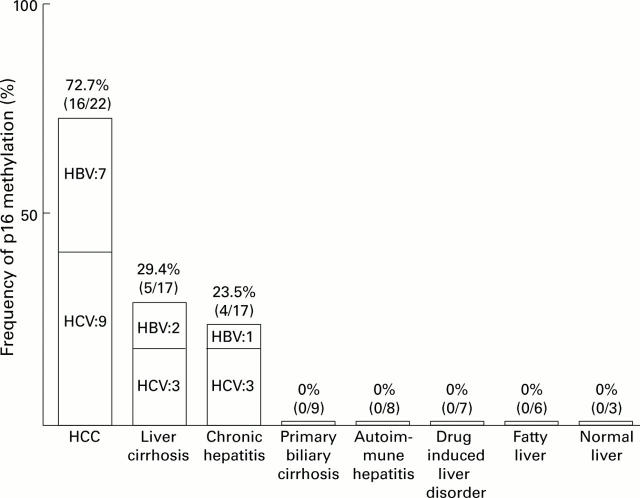
Figure 2 .
Summary of the frequencies of p16 promoter methylation. The frequency of the p16 promoter methylation is expressed as a percentage. The number of positive cases per total number of cases is also shown.
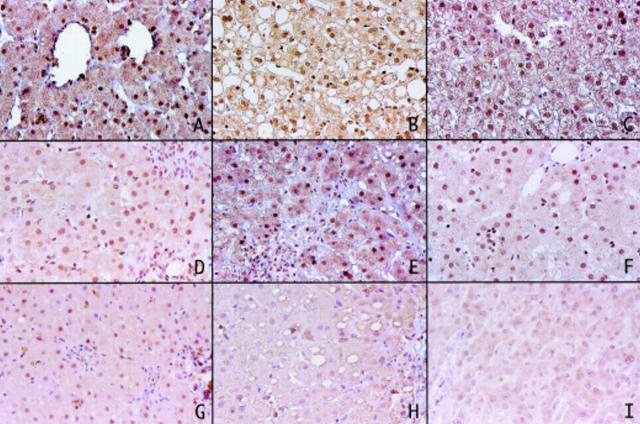
Figure 3 .
Immunohistochemistry of p16 using the mouse anti-p16 monoclonal antibody. Strong nuclear immunostaining was detected in normal liver (A), fatty liver (B), drug induced liver disease (C), autoimmune hepatitis (D), and primary biliary cirrhosis (E) samples. (F) and (G) Partial loss of p16 expression in chronic hepatitis samples. Complete loss of p16 expression in cirrhosis (H) and hepatocellular carcinoma (I) samples.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylin S. B., Herman J. G., Graff J. R., Vertino P. M., Issa J. P. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res. 1998;72:141–196. [PubMed] [Google Scholar]
- Belinsky S. A., Nikula K. J., Palmisano W. A., Michels R., Saccomanno G., Gabrielson E., Baylin S. B., Herman J. G. Aberrant methylation of p16(INK4a) is an early event in lung cancer and a potential biomarker for early diagnosis. Proc Natl Acad Sci U S A. 1998 Sep 29;95(20):11891–11896. doi: 10.1073/pnas.95.20.11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biden K., Young J., Buttenshaw R., Searle J., Cooksley G., Xu D. B., Leggett B. Frequency of mutation and deletion of the tumor suppressor gene CDKN2A (MTS1/p16) in hepatocellular carcinoma from an Australian population. Hepatology. 1997 Mar;25(3):593–597. doi: 10.1002/hep.510250317. [DOI] [PubMed] [Google Scholar]
- Bonilla F., Orlow I., Cordón-Cardó C. Mutational study of p16CDKN2/MTS1/INK4A and p57KIP2 genes in hepatocellular carcinoma. Int J Oncol. 1998 Mar;12(3):583–588. doi: 10.3892/ijo.12.3.583. [DOI] [PubMed] [Google Scholar]
- Chang J., Yang S. H., Cho Y. G., Hwang S. B., Hahn Y. S., Sung Y. C. Hepatitis C virus core from two different genotypes has an oncogenic potential but is not sufficient for transforming primary rat embryo fibroblasts in cooperation with the H-ras oncogene. J Virol. 1998 Apr;72(4):3060–3065. doi: 10.1128/jvi.72.4.3060-3065.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaubert P., Gayer R., Zimmermann A., Fontolliet C., Stamm B., Bosman F., Shaw P. Germ-line mutations of the p16INK4(MTS1) gene occur in a subset of patients with hepatocellular carcinoma. Hepatology. 1997 Jun;25(6):1376–1381. doi: 10.1002/hep.510250613. [DOI] [PubMed] [Google Scholar]
- Foster S. A., Wong D. J., Barrett M. T., Galloway D. A. Inactivation of p16 in human mammary epithelial cells by CpG island methylation. Mol Cell Biol. 1998 Apr;18(4):1793–1801. doi: 10.1128/mcb.18.4.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalgo M. L., Hayashida T., Bender C. M., Pao M. M., Tsai Y. C., Gonzales F. A., Nguyen H. D., Nguyen T. T., Jones P. A. The role of DNA methylation in expression of the p19/p16 locus in human bladder cancer cell lines. Cancer Res. 1998 Mar 15;58(6):1245–1252. [PubMed] [Google Scholar]
- Herman J. G., Graff J. R., Myöhänen S., Nelkin B. D., Baylin S. B. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A. 1996 Sep 3;93(18):9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J. G. p16(INK4): involvement early and often in gastrointestinal malignancies. Gastroenterology. 1999 Feb;116(2):483–485. doi: 10.1016/s0016-5085(99)70146-0. [DOI] [PubMed] [Google Scholar]
- Hsieh C. J., Klump B., Holzmann K., Borchard F., Gregor M., Porschen R. Hypermethylation of the p16INK4a promoter in colectomy specimens of patients with long-standing and extensive ulcerative colitis. Cancer Res. 1998 Sep 1;58(17):3942–3945. [PubMed] [Google Scholar]
- Huang T. H., Perry M. R., Laux D. E. Methylation profiling of CpG islands in human breast cancer cells. Hum Mol Genet. 1999 Mar;8(3):459–470. doi: 10.1093/hmg/8.3.459. [DOI] [PubMed] [Google Scholar]
- Hui A. M., Sakamoto M., Kanai Y., Ino Y., Gotoh M., Yokota J., Hirohashi S. Inactivation of p16INK4 in hepatocellular carcinoma. Hepatology. 1996 Sep;24(3):575–579. doi: 10.1002/hep.510240319. [DOI] [PubMed] [Google Scholar]
- Kamb A. Cell-cycle regulators and cancer. Trends Genet. 1995 Apr;11(4):136–140. doi: 10.1016/s0168-9525(00)89027-7. [DOI] [PubMed] [Google Scholar]
- Kita R., Nishida N., Fukuda Y., Azechi H., Matsuoka Y., Komeda T., Sando T., Nakao K., Ishizaki K. Infrequent alterations of the p16INK4A gene in liver cancer. Int J Cancer. 1996 Jul 17;67(2):176–180. doi: 10.1002/(SICI)1097-0215(19960717)67:2<176::AID-IJC4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Klump B., Hsieh C. J., Holzmann K., Gregor M., Porschen R. Hypermethylation of the CDKN2/p16 promoter during neoplastic progression in Barrett's esophagus. Gastroenterology. 1998 Dec;115(6):1381–1386. doi: 10.1016/s0016-5085(98)70016-2. [DOI] [PubMed] [Google Scholar]
- Liew C. T., Li H. M., Lo K. W., Leow C. K., Chan J. Y., Hin L. Y., Lau W. Y., Lai P. B., Lim B. K., Huang J. High frequency of p16INK4A gene alterations in hepatocellular carcinoma. Oncogene. 1999 Jan 21;18(3):789–795. doi: 10.1038/sj.onc.1202359. [DOI] [PubMed] [Google Scholar]
- Lin Y. W., Chen C. H., Huang G. T., Lee P. H., Wang J. T., Chen D. S., Lu F. J., Sheu J. C. Infrequent mutations and no methylation of CDKN2A (P16/MTS1) and CDKN2B (p15/MTS2) in hepatocellular carcinoma in Taiwan. Eur J Cancer. 1998 Oct;34(11):1789–1795. doi: 10.1016/s0959-8049(98)00189-0. [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Ichida T., Matsuzawa J., Sugimura K., Asakura H. p16(INK4) is inactivated by extensive CpG methylation in human hepatocellular carcinoma. Gastroenterology. 1999 Feb;116(2):394–400. doi: 10.1016/s0016-5085(99)70137-x. [DOI] [PubMed] [Google Scholar]
- Mikovits J. A., Young H. A., Vertino P., Issa J. P., Pitha P. M., Turcoski-Corrales S., Taub D. D., Petrow C. L., Baylin S. B., Ruscetti F. W. Infection with human immunodeficiency virus type 1 upregulates DNA methyltransferase, resulting in de novo methylation of the gamma interferon (IFN-gamma) promoter and subsequent downregulation of IFN-gamma production. Mol Cell Biol. 1998 Sep;18(9):5166–5177. doi: 10.1128/mcb.18.9.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K. Hepatocellular carcinoma: recent progress. Hepatology. 1992 May;15(5):948–963. doi: 10.1002/hep.1840150532. [DOI] [PubMed] [Google Scholar]
- Piao Z., Park C., Lee J. S., Yang C. H., Choi K. Y., Kim H. Homozygous deletions of the CDKN2 gene and loss of heterozygosity of 9p in primary hepatocellular carcinoma. Cancer Lett. 1998 Jan 9;122(1-2):201–207. doi: 10.1016/s0304-3835(97)00403-5. [DOI] [PubMed] [Google Scholar]
- Ray R. B., Lagging L. M., Meyer K., Ray R. Hepatitis C virus core protein cooperates with ras and transforms primary rat embryo fibroblasts to tumorigenic phenotype. J Virol. 1996 Jul;70(7):4438–4443. doi: 10.1128/jvi.70.7.4438-4443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M., Lin A. W., McCurrach M. E., Beach D., Lowe S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997 Mar 7;88(5):593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Sun L., Hui A. M., Kanai Y., Sakamoto M., Hirohashi S. Increased DNA methyltransferase expression is associated with an early stage of human hepatocarcinogenesis. Jpn J Cancer Res. 1997 Dec;88(12):1165–1170. doi: 10.1111/j.1349-7006.1997.tb00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. W., Forrester K., Yeh H., Feitelson M. A., Gu J. R., Harris C. C. Hepatitis B virus X protein inhibits p53 sequence-specific DNA binding, transcriptional activity, and association with transcription factor ERCC3. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2230–2234. doi: 10.1073/pnas.91.6.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilentz R. E., Geradts J., Maynard R., Offerhaus G. J., Kang M., Goggins M., Yeo C. J., Kern S. E., Hruban R. H. Inactivation of the p16 (INK4A) tumor-suppressor gene in pancreatic duct lesions: loss of intranuclear expression. Cancer Res. 1998 Oct 15;58(20):4740–4744. [PubMed] [Google Scholar]
- Wong I. H., Lo Y. M., Zhang J., Liew C. T., Ng M. H., Wong N., Lai P. B., Lau W. Y., Hjelm N. M., Johnson P. J. Detection of aberrant p16 methylation in the plasma and serum of liver cancer patients. Cancer Res. 1999 Jan 1;59(1):71–73. [PubMed] [Google Scholar]
- Zhou P., Jiang W., Weghorst C. M., Weinstein I. B. Overexpression of cyclin D1 enhances gene amplification. Cancer Res. 1996 Jan 1;56(1):36–39. [PubMed] [Google Scholar]