Abstract
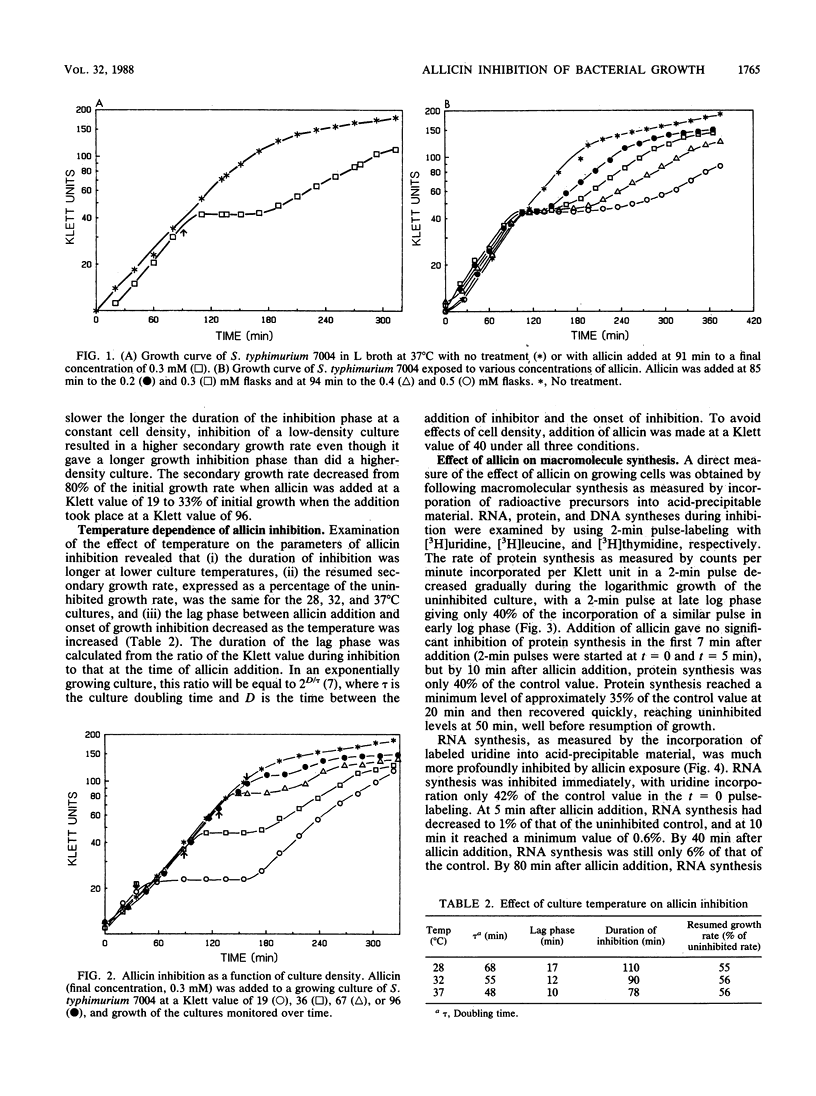
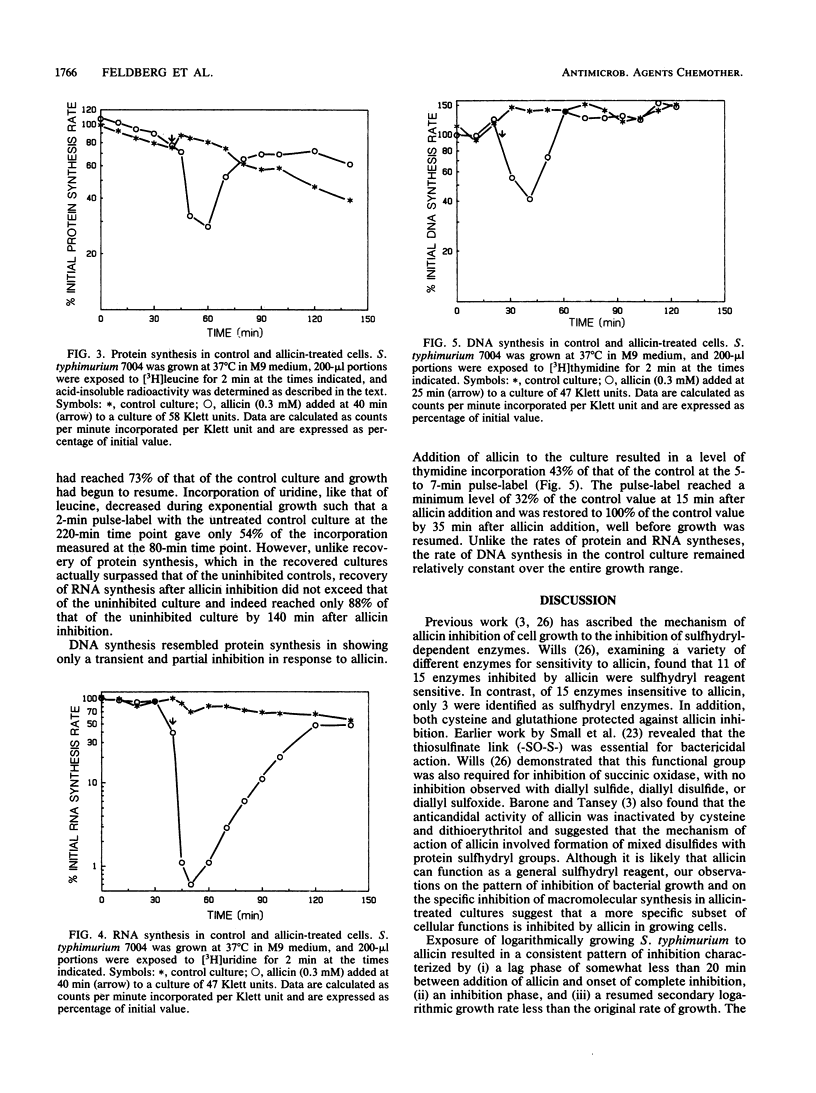
Diallyl thiosulfinate (allicin) is the agent found in garlic which is responsible for the antibacterial and antifungal activity of extracts of this plant. The effect of bacteriostatic concentrations of allicin (0.2 to 0.5 mM) on the growth of Salmonella typhimurium revealed a pattern of inhibition characterized by: (i) a lag of approximately 15 min between addition of allicin and onset of inhibition, (ii) a transitory inhibition phase whose duration was proportional to allicin concentration and inversely proportional to culture density, (iii) a resumed growth phase which showed a lower rate of growth than in uninhibited controls, and (iv) an entry into stationary phase at a lower culture density. Whereas DNA and protein syntheses showed a delayed and partial inhibition by allicin, inhibition of RNA synthesis was immediate and total, suggesting that this is the primary target of allicin action.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleton J. A., Tansey M. R. Inhibition of growth of zoopathogenic fungi by garlic extract. Mycologia. 1975 Jul-Aug;67(4):882–885. [PubMed] [Google Scholar]
- Balandrin M. F., Klocke J. A., Wurtele E. S., Bollinger W. H. Natural plant chemicals: sources of industrial and medicinal materials. Science. 1985 Jun 7;228(4704):1154–1160. doi: 10.1126/science.3890182. [DOI] [PubMed] [Google Scholar]
- Barone F. E., Tansey M. R. Isolation, purification, identification, synthesis, and kinetics of activity of the anticandidal component of Allium sativum, and a hypothesis for its mode of action. Mycologia. 1977 Jul-Aug;69(4):793–825. [PubMed] [Google Scholar]
- Block E. The chemistry of garlic and onions. Sci Am. 1985 Mar;252(3):114–119. doi: 10.1038/scientificamerican0385-114. [DOI] [PubMed] [Google Scholar]
- Bremer H., Chuang L. Cell division after inhibition of chromosome replication in Escherichia coli. J Theor Biol. 1981 Dec 21;93(4):909–926. doi: 10.1016/0022-5193(81)90347-7. [DOI] [PubMed] [Google Scholar]
- Fromtling R. A., Bulmer G. S. In vitro effect of aqueous extract of garlic (Allium sativum) on the growth and viability of Cryptococcus neoformans. Mycologia. 1978 Mar-Apr;70(2):397–405. [PubMed] [Google Scholar]
- Helmstetter C., Cooper S., Pierucci O., Revelas E. On the bacterial life sequence. Cold Spring Harb Symp Quant Biol. 1968;33:809–822. doi: 10.1101/sqb.1968.033.01.093. [DOI] [PubMed] [Google Scholar]
- Katsuki H., Yoshida T., Tanegashima C., Tanaka S. Improved direct method for determination of keto acids by 2,4-dinitrophenylhydrazine. Anal Biochem. 1971 Oct;43(2):349–356. doi: 10.1016/0003-2697(71)90263-6. [DOI] [PubMed] [Google Scholar]
- Moore G. S., Atkins R. D. The fungicidal and fungistatic effects of an aqueous garlic extract on medically important yeast-like fungi. Mycologia. 1977 Mar-Apr;69(2):341–348. [PubMed] [Google Scholar]
- Nakata C., Nakata T., Hishikawa A. An improved colorimetric determination of thiolsulfinate. Anal Biochem. 1970 Sep;37(1):92–97. doi: 10.1016/0003-2697(70)90262-9. [DOI] [PubMed] [Google Scholar]
- Nock L. P., Mazelis M. The C-S Lyases of Higher Plants : Direct Comparison of the Physical Properties of Homogeneous Alliin Lyase of Garlic (Allium sativum) and Onion (Allium cepa). Plant Physiol. 1987 Dec;85(4):1079–1083. doi: 10.1104/pp.85.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock L. P., Mazelis M. The C-S lyases of higher plants: preparation and properties of homogeneous alliin lyase from garlic (Allium sativum). Arch Biochem Biophys. 1986 Aug 15;249(1):27–33. doi: 10.1016/0003-9861(86)90556-4. [DOI] [PubMed] [Google Scholar]
- SCHWIMMER S., RYAN C. A., WONG F. F. SPECIFICITY OF L-CYSTEINE SULFOXIDE LYASE AND PARTIALLY COMPETITIVE INHIBITION BY S-ALKYL-L-CYSTEINES. J Biol Chem. 1964 Mar;239:777–782. [PubMed] [Google Scholar]
- Schwimmer S. Characterization of S-propenyl-L-cysteine sulfoxide as the principal endogenous substrate of L-cysteine sulfoxide lyase of onion. Arch Biochem Biophys. 1969 Mar;130(1):312–320. doi: 10.1016/0003-9861(69)90038-1. [DOI] [PubMed] [Google Scholar]
- Tobkin H. E., Jr, Mazelis M. Alliin lyase: preparation and characterization of the homogeneous enzyme from onion bulbs. Arch Biochem Biophys. 1979 Mar;193(1):150–157. doi: 10.1016/0003-9861(79)90018-3. [DOI] [PubMed] [Google Scholar]
- WILLS E. D. Enzyme inhibition by allicin, the active principle of garlic. Biochem J. 1956 Jul;63(3):514–520. doi: 10.1042/bj0630514. [DOI] [PMC free article] [PubMed] [Google Scholar]



