Abstract
BACKGROUND—Selective inhibitors of cyclooxygenase (COX)-2 may provoke less gastric damage and platelet inhibition than conventional non-steroidal anti-inflammatory drugs. AIMS—We compared the biochemical and gastrointestinal effects of nimesulide, a potent and selective COX-2 inhibitor, with naproxen which exhibits no selectivity. SUBJECTS—Thirty six healthy volunteers were randomised to nimesulide 100 mg or naproxen 500 mg twice daily for two weeks in a double blind, crossover study with a washout between treatments. METHODS—Gastrointestinal side effects were assessed by endoscopy, and by estimation of small intestinal absorption-permeability and inflammation. Comparisons were made between variables at the end of each treatment phase. RESULTS—Nimesulide caused significantly less gastric injury using the modified Lanza score (p<0.001) as well as reduced duodenum injury (p=0.039). Nimesulide had lower visual analogue scores (VAS) for haemorrhage and erosive lesions in the stomach (p<0.001) and for mucosal injection in the duodenum (p=0.039). Naproxen increased excretion of calprotectin, a marker of intestinal inflammation (5.5 (1.2) to 12.1 (2.1) mg/l) while nimesulide had no effect (treatment difference p=0.03). Naproxen abolished platelet aggregation to arachidonic acid and suppressed serum thromboxane B2 (TXB2) by 98%, indices of COX-1 activity. In contrast, nimesulide had no significant effect on platelet aggregation, although it reduced serum TXB2 by 29%. Production of prostaglandin E2 and prostacyclin by gastric biopsies, also COX-1 dependent, was inhibited by naproxen, but not by nimesulide. COX-2 activity, determined as endotoxin induced prostaglandin E2 formation in plasma, was markedly suppressed by both treatments. INTERPRETATION—Nimesulide has preferential selectivity for COX-2 over COX-1 in vivo at full therapeutic doses and induces less gastrointestinal damage than that seen with naproxen in the short term. Keywords: cyclooxygenase; prostaglandins; platelet aggregation; non-steroidal anti-inflammatory drug enteropathy
Full Text
The Full Text of this article is available as a PDF (185.5 KB).
Figure 1 .
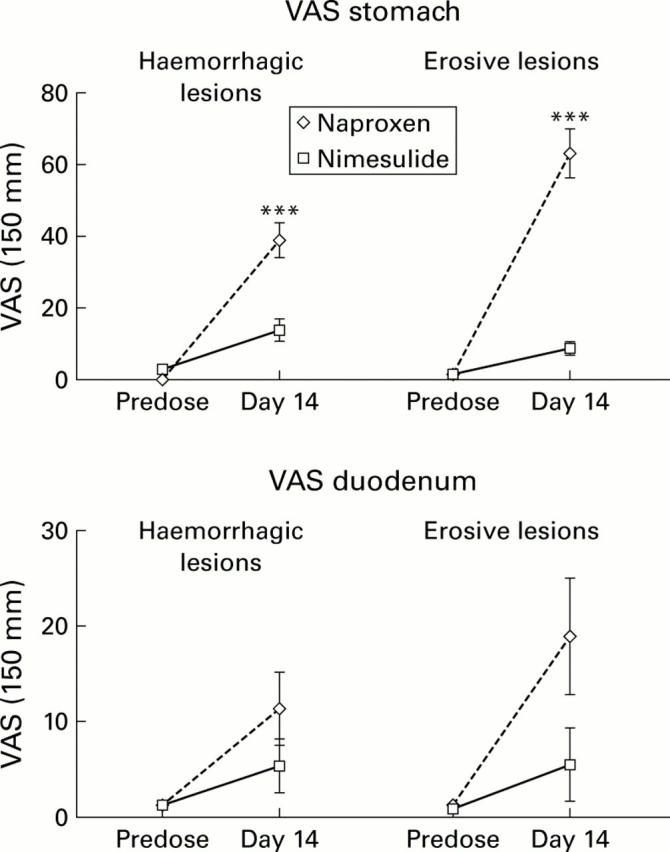
Comparison of the effects of nimesulide and naproxen on visual analogue scores (VAS) at endoscopy of the upper gastrointestinal tract before and during treatment with nimesulide 100 mg twice daily or naproxen 500 mg twice daily. Data are shown as mean (SEM). ***p<0.001 between treatments.
Figure 2 .
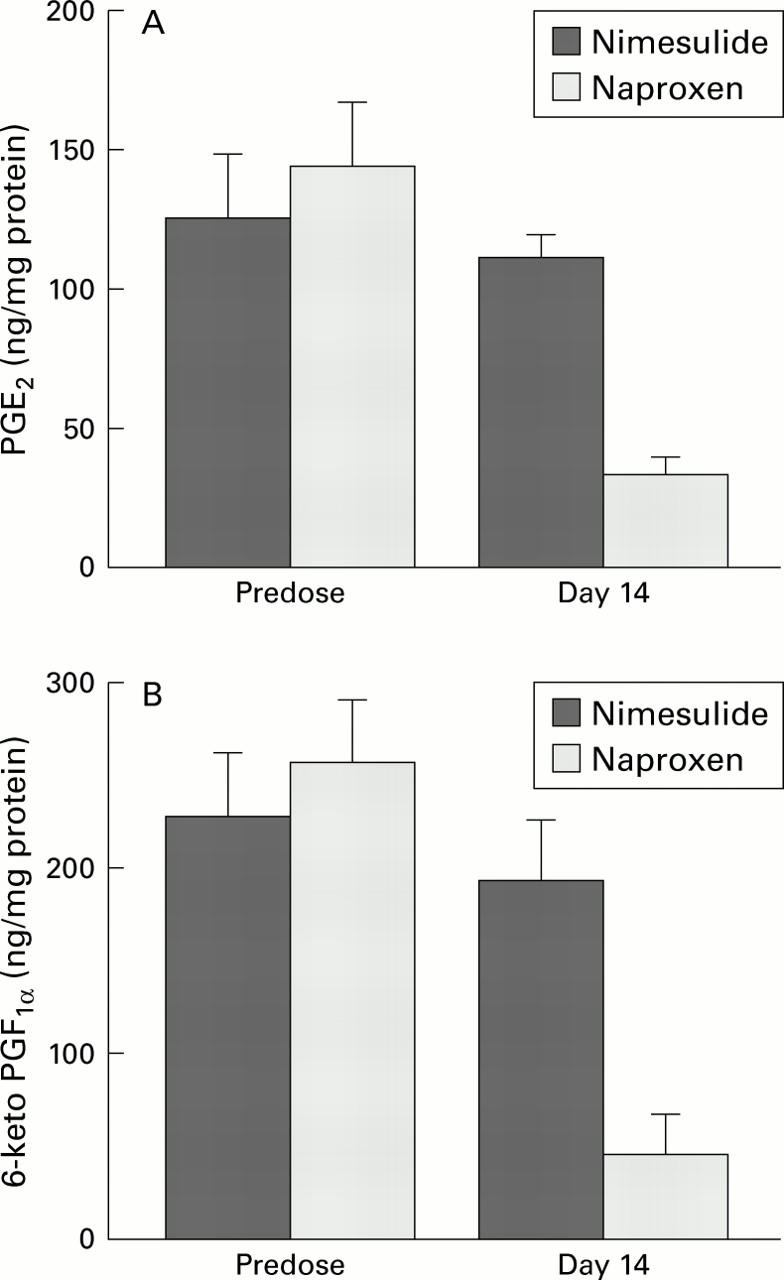
Comparison of the effects of nimesulide and naproxen on generation of prostaglandin (PG) E2 (A) and 6-keto-PGF1α (B) by gastric biopsies incubated at 37°C for 45 minutes before and during treatment with nimesulide 100 mg twice daily or naproxen 500 mg twice daily. Data are shown as mean (SEM). p<0.01 between treatments.
Figure 3 .
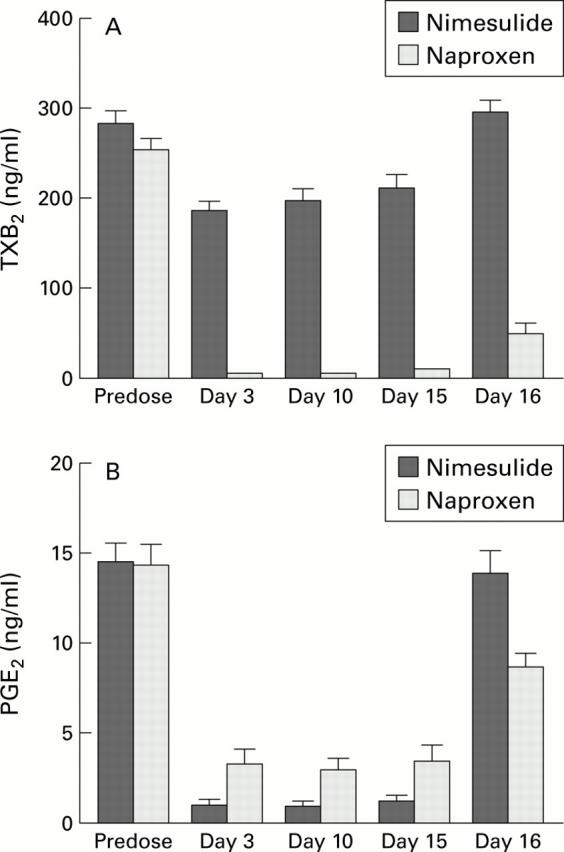
(A) Comparison of the effects of nimesulide and naproxen on serum thromboxane B2 (TXB2) (a measure of COX-1 activity) before and during treatment with nimesulide 100 mg twice daily or naproxen 500 mg twice daily. Data are shown as mean (SEM). Naproxen had a greater effect on serum TXB2 (p<0.001 for comparison between treatments to day 10). Note that nimesulide reduced serum TxB2 on average by 29% from baseline (p<0.01) and that both drugs markedly reduced plasma PGE2. (B) Plasma prostaglandin (PG) E2 (a measure of COX-2 activity). Nimesulide had a slightly greater effect on plasma PGE2 (p=0.053 for comparison between treatments to day 10).
Figure 4 .
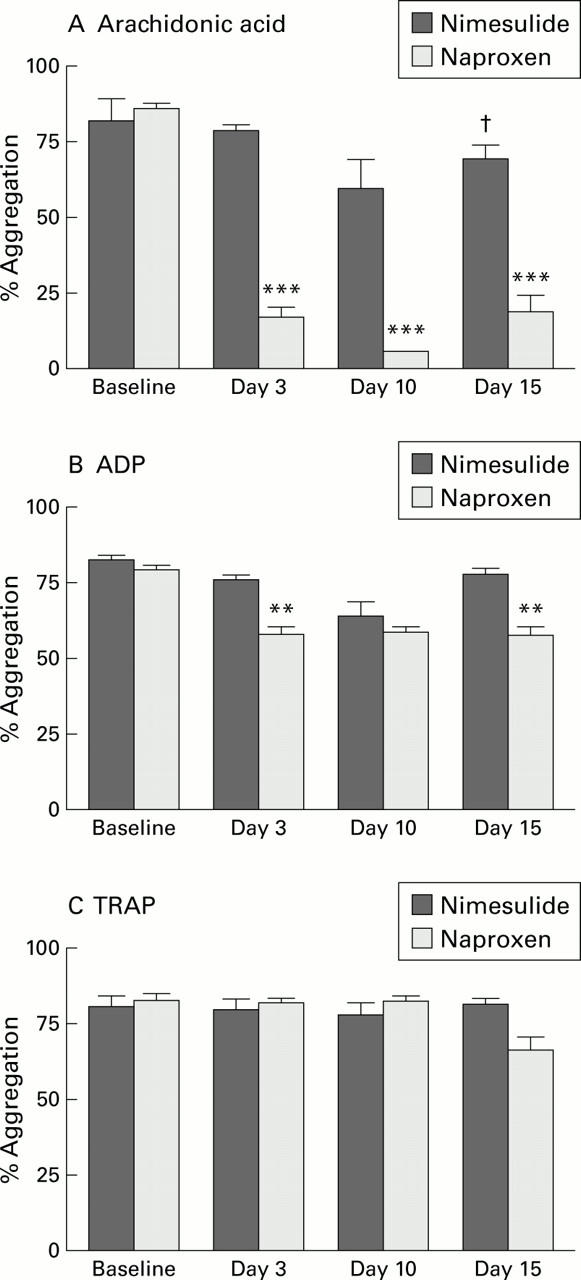
Comparison of the effects of nimesulide and naproxen on platelet aggregation to arachidonic acid (A), adenosine diphosphate (ADP) (B), and thrombin receptor activator peptide (TRAP) (C) before and during treatment with nimesulide 100 mg twice daily or naproxen 500 mg twice daily. Data are shown as mean (SEM). **p<0.01, ***p<0.001 for changes from baseline; †p=0.048 for comparison between treatments.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aabakken L., Larsen S., Osnes M. Visual analogue scales for endoscopic evaluation of nonsteroidal anti-inflammatory drug-induced mucosal damage in the stomach and duodenum. Scand J Gastroenterol. 1990 May;25(5):443–448. doi: 10.3109/00365529009095513. [DOI] [PubMed] [Google Scholar]
- Aabakken L., Osnes M. 51Cr-ethylenediaminetetraacetic acid absorption test. Effects of naproxen, a non-steroidal, antiinflammatory drug. Scand J Gastroenterol. 1990 Sep;25(9):917–924. doi: 10.3109/00365529008997613. [DOI] [PubMed] [Google Scholar]
- Armstrong C. P., Blower A. L. Non-steroidal anti-inflammatory drugs and life threatening complications of peptic ulceration. Gut. 1987 May;28(5):527–532. doi: 10.1136/gut.28.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardhan K. D., Bjarnason I., Scott D. L., Griffin W. M., Fenn G. C., Shield M. J., Morant S. V. The prevention and healing of acute non-steroidal anti-inflammatory drug-associated gastroduodenal mucosal damage by misoprostol. Br J Rheumatol. 1993 Nov;32(11):990–995. doi: 10.1093/rheumatology/32.11.990. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Hayllar J., MacPherson A. J., Russell A. S. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology. 1993 Jun;104(6):1832–1847. doi: 10.1016/0016-5085(93)90667-2. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., MacPherson A., Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995 May;108(5):1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Macpherson A., Rotman H., Schupp J., Hayllar J. A randomized, double-blind, crossover comparative endoscopy study on the gastroduodenal tolerability of a highly specific cyclooxygenase-2 inhibitor, flosulide, and naproxen. Scand J Gastroenterol. 1997 Feb;32(2):126–130. doi: 10.3109/00365529709000182. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Price A. B., Zanelli G., Smethurst P., Burke M., Gumpel J. M., Levi A. J. Clinicopathological features of nonsteroidal antiinflammatory drug-induced small intestinal strictures. Gastroenterology. 1988 Apr;94(4):1070–1074. doi: 10.1016/0016-5085(88)90568-9. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Zanelli G., Prouse P., Smethurst P., Smith T., Levi S., Gumpel M. J., Levi A. J. Blood and protein loss via small-intestinal inflammation induced by non-steroidal anti-inflammatory drugs. Lancet. 1987 Sep 26;2(8561):711–714. doi: 10.1016/s0140-6736(87)91075-0. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Zanelli G., Smith T., Prouse P., Williams P., Smethurst P., Delacey G., Gumpel M. J., Levi A. J. Nonsteroidal antiinflammatory drug-induced intestinal inflammation in humans. Gastroenterology. 1987 Sep;93(3):480–489. doi: 10.1016/0016-5085(87)90909-7. [DOI] [PubMed] [Google Scholar]
- Bode C., Maute G., Bode J. C. Prostaglandin E2 and prostaglandin F2 alpha biosynthesis in human gastric mucosa: effect of chronic alcohol misuse. Gut. 1996 Sep;39(3):348–352. doi: 10.1136/gut.39.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. C., Boyce S., Brideau C., Ford-Hutchinson A. W., Gordon R., Guay D., Hill R. G., Li C. S., Mancini J., Penneton M. Pharmacology of a selective cyclooxygenase-2 inhibitor, L-745,337: a novel nonsteroidal anti-inflammatory agent with an ulcerogenic sparing effect in rat and nonhuman primate stomach. J Pharmacol Exp Ther. 1995 Sep;274(3):1531–1537. [PubMed] [Google Scholar]
- Cullen D. J., Hawkey G. M., Greenwood D. C., Humphreys H., Shepherd V., Logan R. F., Hawkey C. J. Peptic ulcer bleeding in the elderly: relative roles of Helicobacter pylori and non-steroidal anti-inflammatory drugs. Gut. 1997 Oct;41(4):459–462. doi: 10.1136/gut.41.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen L., Kelly L., Connor S. O., Fitzgerald D. J. Selective cyclooxygenase-2 inhibition by nimesulide in man. J Pharmacol Exp Ther. 1998 Nov;287(2):578–582. [PubMed] [Google Scholar]
- Dale I., Brandtzaeg P., Fagerhol M. K., Scott H. Distribution of a new myelomonocytic antigen (L1) in human peripheral blood leukocytes. Immunofluorescence and immunoperoxidase staining features in comparison with lysozyme and lactoferrin. Am J Clin Pathol. 1985 Jul;84(1):24–34. doi: 10.1093/ajcp/84.1.24. [DOI] [PubMed] [Google Scholar]
- DeWitt D. L., Meade E. A. Serum and glucocorticoid regulation of gene transcription and expression of the prostaglandin H synthase-1 and prostaglandin H synthase-2 isozymes. Arch Biochem Biophys. 1993 Oct;306(1):94–102. doi: 10.1006/abbi.1993.1485. [DOI] [PubMed] [Google Scholar]
- Dixon M. F., Genta R. M., Yardley J. H., Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996 Oct;20(10):1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- FitzGerald G. A., Brash A. R., Falardeau P., Oates J. A. Estimated rate of prostacyclin secretion into the circulation of normal man. J Clin Invest. 1981 Nov;68(5):1272–1276. doi: 10.1172/JCI110373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S., Ramanujam K. S., Wong A., Fantry G. T., Drachenberg C. B., James S. P., Meltzer S. J., Wilson K. T. Increased expression and cellular localization of inducible nitric oxide synthase and cyclooxygenase 2 in Helicobacter pylori gastritis. Gastroenterology. 1999 Jun;116(6):1319–1329. doi: 10.1016/s0016-5085(99)70496-8. [DOI] [PubMed] [Google Scholar]
- Gabriel S. E., Jaakkimainen L., Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. A meta-analysis. Ann Intern Med. 1991 Nov 15;115(10):787–796. doi: 10.7326/0003-4819-115-10-787. [DOI] [PubMed] [Google Scholar]
- Hawkey C., Laine L., Simon T., Beaulieu A., Maldonado-Cocco J., Acevedo E., Shahane A., Quan H., Bolognese J., Mortensen E. Comparison of the effect of rofecoxib (a cyclooxygenase 2 inhibitor), ibuprofen, and placebo on the gastroduodenal mucosa of patients with osteoarthritis: a randomized, double-blind, placebo-controlled trial. The Rofecoxib Osteoarthritis Endoscopy Multinational Study Group. Arthritis Rheum. 2000 Feb;43(2):370–377. doi: 10.1002/1529-0131(200002)43:2<370::AID-ANR17>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Jones D. A., Carlton D. P., McIntyre T. M., Zimmerman G. A., Prescott S. M. Molecular cloning of human prostaglandin endoperoxide synthase type II and demonstration of expression in response to cytokines. J Biol Chem. 1993 Apr 25;268(12):9049–9054. [PubMed] [Google Scholar]
- Kargman S., Charleson S., Cartwright M., Frank J., Riendeau D., Mancini J., Evans J., O'Neill G. Characterization of Prostaglandin G/H Synthase 1 and 2 in rat, dog, monkey, and human gastrointestinal tracts. Gastroenterology. 1996 Aug;111(2):445–454. doi: 10.1053/gast.1996.v111.pm8690211. [DOI] [PubMed] [Google Scholar]
- Khanna I. K., Weier R. M., Yu Y., Collins P. W., Miyashiro J. M., Koboldt C. M., Veenhuizen A. W., Currie J. L., Seibert K., Isakson P. C. 1,2-Diarylpyrroles as potent and selective inhibitors of cyclooxygenase-2. J Med Chem. 1997 May 23;40(11):1619–1633. doi: 10.1021/jm970036a. [DOI] [PubMed] [Google Scholar]
- Koch G. G. The use of non-parametric methods in the statistical analysis of the two-period change-over design. Biometrics. 1972 Jun;28(2):577–584. [PubMed] [Google Scholar]
- Laneuville O., Breuer D. K., Dewitt D. L., Hla T., Funk C. D., Smith W. L. Differential inhibition of human prostaglandin endoperoxide H synthases-1 and -2 by nonsteroidal anti-inflammatory drugs. J Pharmacol Exp Ther. 1994 Nov;271(2):927–934. [PubMed] [Google Scholar]
- Langenbach R., Morham S. G., Tiano H. F., Loftin C. D., Ghanayem B. I., Chulada P. C., Mahler J. F., Lee C. A., Goulding E. H., Kluckman K. D. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995 Nov 3;83(3):483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- Langman M. J. Epidemiologic evidence on the association between peptic ulceration and antiinflammatory drug use. Gastroenterology. 1989 Feb;96(2 Pt 2 Suppl):640–646. doi: 10.1016/s0016-5085(89)80060-5. [DOI] [PubMed] [Google Scholar]
- Lanza F. L. Endoscopic studies of gastric and duodenal injury after the use of ibuprofen, aspirin, and other nonsteroidal anti-inflammatory agents. Am J Med. 1984 Jul 13;77(1A):19–24. doi: 10.1016/s0002-9343(84)80014-5. [DOI] [PubMed] [Google Scholar]
- Lanza F., Rack M. F., Lynn M., Wolf J., Sanda M. An endoscopic comparison of the effects of etodolac, indomethacin, ibuprofen, naproxen, and placebo on the gastrointestinal mucosa. J Rheumatol. 1987 Apr;14(2):338–341. [PubMed] [Google Scholar]
- Lim S. G., Menzies I. S., Lee C. A., Johnson M. A., Pounder R. E. Intestinal permeability and function in patients infected with human immunodeficiency virus. A comparison with coeliac disease. Scand J Gastroenterol. 1993 Jul;28(7):573–580. doi: 10.3109/00365529309096090. [DOI] [PubMed] [Google Scholar]
- Lockard O. O., Jr, Ivey K. J., Butt J. H., Silvoso G. R., Sisk C., Holt S. The prevalence of duodenal lesions in patients with rheumatic diseases on chronic aspirin therapy. Gastrointest Endosc. 1980 Feb;26(1):5–7. doi: 10.1016/s0016-5107(80)73248-0. [DOI] [PubMed] [Google Scholar]
- MacDonald T. M., Morant S. V., Robinson G. C., Shield M. J., McGilchrist M. M., Murray F. E., McDevitt D. G. Association of upper gastrointestinal toxicity of non-steroidal anti-inflammatory drugs with continued exposure: cohort study. BMJ. 1997 Nov 22;315(7119):1333–1337. doi: 10.1136/bmj.315.7119.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masferrer J. L., Zweifel B. S., Manning P. T., Hauser S. D., Leahy K. M., Smith W. G., Isakson P. C., Seibert K. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3228–3232. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi N., Yamada A., Hiraishi M., Konishi T., Minota S., Saito T., Sugano K., Yazaki Y., Mori M., Shiga J. Multiple strictures of the small intestine after long-term nonsteroidal anti-inflammatory drug therapy. Am J Gastroenterol. 1992 Sep;87(9):1183–1186. [PubMed] [Google Scholar]
- McAdam B. F., Catella-Lawson F., Mardini I. A., Kapoor S., Lawson J. A., FitzGerald G. A. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci U S A. 1999 Jan 5;96(1):272–277. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meling T. R., Aabakken L., Røseth A., Osnes M. Faecal calprotectin shedding after short-term treatment with non-steroidal anti-inflammatory drugs. Scand J Gastroenterol. 1996 Apr;31(4):339–344. doi: 10.3109/00365529609006407. [DOI] [PubMed] [Google Scholar]
- Menzies I. S., Mount J. N., Wheeler M. J. Quantitative estimation of clinically important monosaccharides in plasma by rapid thin layer chromatography. Ann Clin Biochem. 1978 Mar;15(2):65–76. doi: 10.1177/000456327801500116. [DOI] [PubMed] [Google Scholar]
- Merlie J. P., Fagan D., Mudd J., Needleman P. Isolation and characterization of the complementary DNA for sheep seminal vesicle prostaglandin endoperoxide synthase (cyclooxygenase). J Biol Chem. 1988 Mar 15;263(8):3550–3553. [PubMed] [Google Scholar]
- Panara M. R., Greco A., Santini G., Sciulli M. G., Rotondo M. T., Padovano R., di Giamberardino M., Cipollone F., Cuccurullo F., Patrono C. Effects of the novel anti-inflammatory compounds, N-[2-(cyclohexyloxy)-4-nitrophenyl] methanesulphonamide (NS-398) and 5-methanesulphonamido-6-(2,4-difluorothio-phenyl)-1-inda none (L-745,337), on the cyclo-oxygenase activity of human blood prostaglandin endoperoxide synthases. Br J Pharmacol. 1995 Nov;116(5):2429–2434. doi: 10.1111/j.1476-5381.1995.tb15091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus H. E. FDA Arthritis Advisory Committee meeting: postmarketing surveillance of nonsteroidal antiinflammatory drugs. Arthritis Rheum. 1985 Oct;28(10):1168–1169. doi: 10.1002/art.1780281014. [DOI] [PubMed] [Google Scholar]
- Røseth A. G., Fagerhol M. K., Aadland E., Schjønsby H. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol. 1992 Sep;27(9):793–798. doi: 10.3109/00365529209011186. [DOI] [PubMed] [Google Scholar]
- Seibert K., Zhang Y., Leahy K., Hauser S., Masferrer J., Perkins W., Lee L., Isakson P. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigthorsson G., Jacob M., Wrigglesworth J., Somasundaram S., Tavares I., Foster R., Roseth A., Rafi S., Mahmud T., Simpson R. Comparison of indomethacin and nimesulide, a selective cyclooxygenase-2 inhibitor, on key pathophysiologic steps in the pathogenesis of nonsteroidal anti-inflammatory drug enteropathy in the rat. Scand J Gastroenterol. 1998 Jul;33(7):728–735. doi: 10.1080/00365529850171675. [DOI] [PubMed] [Google Scholar]
- Sigthorsson G., Tibble J., Hayllar J., Menzies I., Macpherson A., Moots R., Scott D., Gumpel M. J., Bjarnason I. Intestinal permeability and inflammation in patients on NSAIDs. Gut. 1998 Oct;43(4):506–511. doi: 10.1136/gut.43.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein F. E., Graham D. Y., Senior J. R., Davies H. W., Struthers B. J., Bittman R. M., Geis G. S. Misoprostol reduces serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995 Aug 15;123(4):241–249. doi: 10.7326/0003-4819-123-4-199508150-00001. [DOI] [PubMed] [Google Scholar]
- Simon L. S., Weaver A. L., Graham D. Y., Kivitz A. J., Lipsky P. E., Hubbard R. C., Isakson P. C., Verburg K. M., Yu S. S., Zhao W. W. Anti-inflammatory and upper gastrointestinal effects of celecoxib in rheumatoid arthritis: a randomized controlled trial. JAMA. 1999 Nov 24;282(20):1921–1928. doi: 10.1001/jama.282.20.1921. [DOI] [PubMed] [Google Scholar]
- Teahon K., Smethurst P., Levi A. J., Menzies I. S., Bjarnason I. Intestinal permeability in patients with Crohn's disease and their first degree relatives. Gut. 1992 Mar;33(3):320–323. doi: 10.1136/gut.33.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane J. R., Mitchell J. A., Appleton I., Tomlinson A., Bishop-Bailey D., Croxtall J., Willoughby D. A. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2046–2050. doi: 10.1073/pnas.91.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. L., Bak A., McKnight W., Asfaha S., Sharkey K. A., MacNaughton W. K. Cyclooxygenase 1 contributes to inflammatory responses in rats and mice: implications for gastrointestinal toxicity. Gastroenterology. 1998 Jul;115(1):101–109. doi: 10.1016/s0016-5085(98)70370-1. [DOI] [PubMed] [Google Scholar]


