Abstract
BACKGROUND—The presence of high level DNA microsatellite instability (MSI-H) in colorectal cancer is associated with an improved prognosis, as is the presence of tumour infiltrating lymphocytes (TILs). It is not clear if TILs contribute directly to the survival advantage associated with MSI-H cancers through activation of an antitumour immune response. AIMS—To correlate TIL and apoptosis rates in colorectal cancer stratified by MSI status. METHODS—The distribution of TILs was characterised and quantified in a selected series of 102 sporadic colorectal cancers classified according to levels of MSI as 32 MSI-H, 30 MSI-low (MSI-L), and 40 microsatellite stable (MSS). Archival blocks were immunostained using the T cell markers CD3 and CD8, and the B cell marker CD20. Apoptosis of malignant epithelial cells was quantified by immunohistochemistry with the M30 CytoDEATH antibody. RESULTS—Positive staining with anti-CD3 and negative staining with anti-CD20 identified virtually all TILs as T cells. The majority of CD3+ TILs (>75%) also stained with anti-CD8. TILs were most abundant in MSI-H colorectal cancers in which 23/32 (72%) scored as TIL positive. Only 5/40 (12.5%) MSS tumours and 9/30 (30%) MSI-L cancers were TIL positive (p<0.0001). MSI-H cancers showed a twofold higher rate of apoptosis (mean (SD) 3.52 (0.34)%) than the MSS cancers (1.53 (0.23)%) while the MSI-L subgroup had an intermediate level (2.52 (0.35)%) (p<0.0001). Overall, there was a small (r=0.347) but significant linear correlation between CD3+ and M30+ random apoptosis counts (p<0.001). However, TILs and apoptosis showed little colocalisation. CONCLUSIONS—While TILs might be expected to explain the increased apoptotic rate and improved prognosis of MSI-H cancers, it is likely that TILs and apoptosis are independent characteristics of MSI-H cancers. Keywords: colorectal cancer; DNA microsatellite instability; tumour infiltrating lymphocytes; apoptosis
Full Text
The Full Text of this article is available as a PDF (362.8 KB).
Figure 1 .
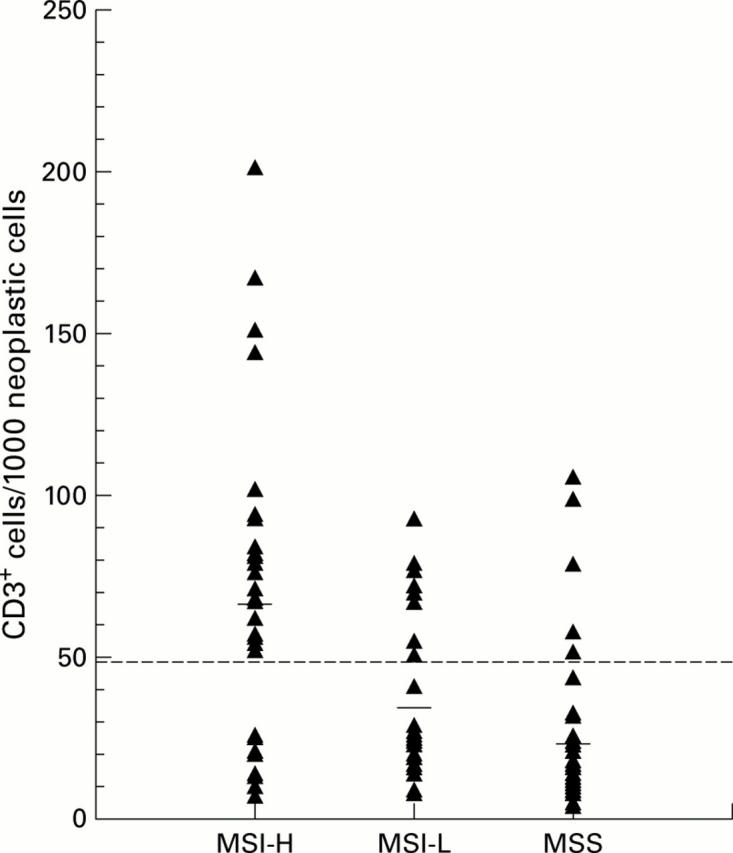
Distribution of CD3+ tumour infiltrating lymphocytes (TILs) in colorectal cancer. All 102 tumour CD3+ counts are represented by a triangle. The score of 50 CD3+ lymphocytes was chosen as the cut off for TIL positive cancers (broken line) and the horizontal bars represent the mean count for each group. MSS, microsatellite stable; MSI-L, microsatellite instability-low; MSI-H, microsatellite instability-high.
Figure 2 .
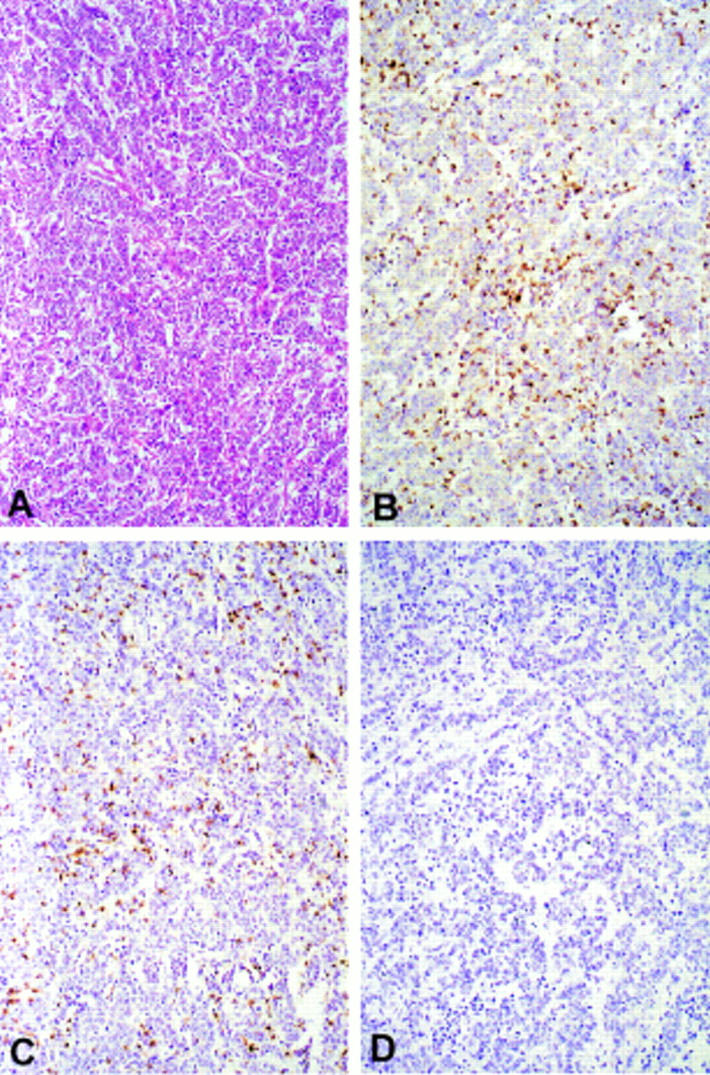
Example of a typical undifferentiated microsatellite instability-high (MSI-H) cancer heavily infiltrated with lymphocytes. (A) Standard haematoxylin and eosin stained section. (B) Serial section of the same tumour stained with anti-CD3, revealing T lymphocytes. (C) Serial section stained with anti-CD8. Note that most CD3+ T cells also show positive staining with anti-CD8. (D) Serial section of the same tumour stained with anti-CD20, a B cell marker. Note that no intraepithelial cells have stained positively in this section (original magnification ×60).
Figure 3 .
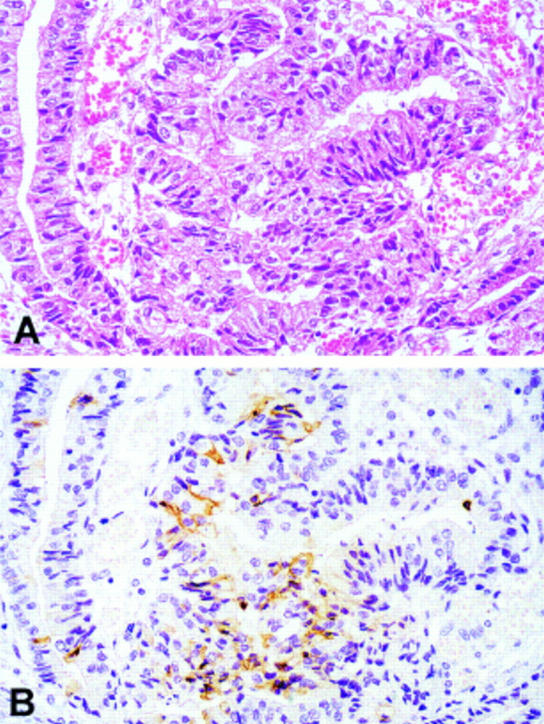
Serial sections of a microsatellite instability-low (MSI-L) cancer (A) stained routinely with haematoxylin and eosin and (B) staining with the M30 cytoDEATH antibody specific for epithelial cell apoptosis. The brown cytoplasmic staining indicates the many apoptotic tumour cells present in this field (original magnification ×120).
Figure 4 .
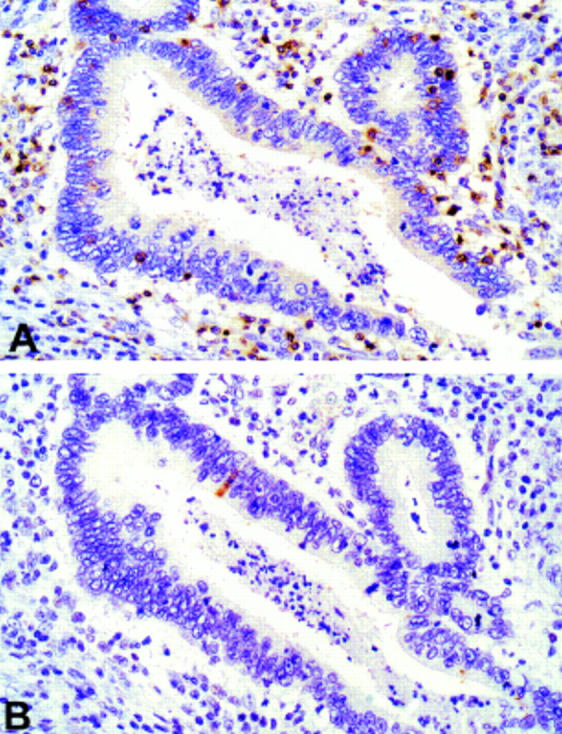
Serial sections of a well differentiated colorectal cancer stained with (A) anti-CD3 and (B) M30 CytoDEATH antibody. Note that only one apoptotic cell is present in this area densely infiltrated with tumour infiltrating lymphocytes (original magnification ×120).
Figure 5 .
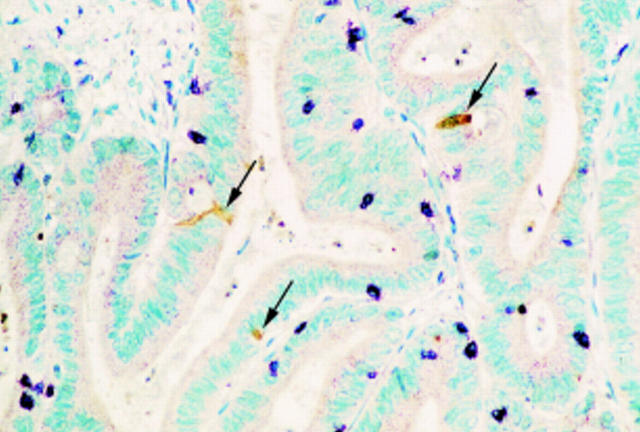
Example of M30 CytoDEATH and CD3 double staining, counterstained with methyl green. The brown DAB staining indicates M30+ apoptotic tumour cells (arrows) and purple Vector VIP staining reveals the CD3+ T cells present in this tumour infiltrating lymphocyte (TIL) positive microsatellite instability-high (MSI-H) cancer. Note that M30+ neoplastic cells and CD3+ TILs do not coincide (original magnification ×120).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biden K. G., Simms L. A., Cummings M., Buttenshaw R., Schoch E., Searle J., Gobe G., Jass J. R., Meltzer S. J., Leggett B. A. Expression of Bcl-2 protein is decreased in colorectal adenocarcinomas with microsatellite instability. Oncogene. 1999 Feb 4;18(5):1245–1249. doi: 10.1038/sj.onc.1202413. [DOI] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Guy-Grand D., Griscelli C. Intraepithelial lymphocytes of human gut: isolation, characterisation and study of natural killer activity. Gut. 1985 Jan;26(1):81–88. doi: 10.1136/gut.26.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. M., Sun X. M., Snowden R. T., Dinsdale D., Skilleter D. N. Key morphological features of apoptosis may occur in the absence of internucleosomal DNA fragmentation. Biochem J. 1992 Sep 1;286(Pt 2):331–334. doi: 10.1042/bj2860331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcetti R., Viel A., Doglioni C., Russo A., Guidoboni M., Capozzi E., Vecchiato N., Macrì E., Fornasarig M., Boiocchi M. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol. 1999 Jun;154(6):1805–1813. doi: 10.1016/S0002-9440(10)65436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enright H., Hebbel R. P., Nath K. A. Internucleosomal cleavage of DNA as the sole criterion for apoptosis may be artifactual. J Lab Clin Med. 1994 Jul;124(1):63–68. [PubMed] [Google Scholar]
- Hirata I., Berrebi G., Austin L. L., Keren D. F., Dobbins W. O., 3rd Immunohistological characterization of intraepithelial and lamina propria lymphocytes in control ileum and colon and in inflammatory bowel disease. Dig Dis Sci. 1986 Jun;31(6):593–603. doi: 10.1007/BF01318690. [DOI] [PubMed] [Google Scholar]
- Jass J. R., Biden K. G., Cummings M. C., Simms L. A., Walsh M., Schoch E., Meltzer S. J., Wright C., Searle J., Young J. Characterisation of a subtype of colorectal cancer combining features of the suppressor and mild mutator pathways. J Clin Pathol. 1999 Jun;52(6):455–460. doi: 10.1136/jcp.52.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jass J. R., Do K. A., Simms L. A., Iino H., Wynter C., Pillay S. P., Searle J., Radford-Smith G., Young J., Leggett B. Morphology of sporadic colorectal cancer with DNA replication errors. Gut. 1998 May;42(5):673–679. doi: 10.1136/gut.42.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y., Dinjens W. N., Bosman F. T. Proliferation and apoptosis in proliferative lesions of the colon and rectum. Virchows Arch. 1997 Aug;431(2):111–117. doi: 10.1007/s004280050076. [DOI] [PubMed] [Google Scholar]
- Kim H., Jen J., Vogelstein B., Hamilton S. R. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol. 1994 Jul;145(1):148–156. [PMC free article] [PubMed] [Google Scholar]
- Koike M. Significance of spontaneous apoptosis during colorectal tumorigenesis. J Surg Oncol. 1996 Jun;62(2):97–108. doi: 10.1002/(SICI)1096-9098(199606)62:2<97::AID-JSO5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Leers M. P., Kölgen W., Björklund V., Bergman T., Tribbick G., Persson B., Björklund P., Ramaekers F. C., Björklund B., Nap M. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. J Pathol. 1999 Apr;187(5):567–572. doi: 10.1002/(SICI)1096-9896(199904)187:5<567::AID-PATH288>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Liu L. U., Holt P. R., Krivosheyev V., Moss S. F. Human right and left colon differ in epithelial cell apoptosis and in expression of Bak, a pro-apoptotic Bcl-2 homologue. Gut. 1999 Jul;45(1):45–50. doi: 10.1136/gut.45.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothe R. A., Peltomäki P., Meling G. I., Aaltonen L. A., Nyström-Lahti M., Pylkkänen L., Heimdal K., Andersen T. I., Møller P., Rognum T. O. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res. 1993 Dec 15;53(24):5849–5852. [PubMed] [Google Scholar]
- Markowitz S., Wang J., Myeroff L., Parsons R., Sun L., Lutterbaugh J., Fan R. S., Zborowska E., Kinzler K. W., Vogelstein B. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995 Jun 2;268(5215):1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- Naito Y., Saito K., Shiiba K., Ohuchi A., Saigenji K., Nagura H., Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998 Aug 15;58(16):3491–3494. [PubMed] [Google Scholar]
- Rampino N., Yamamoto H., Ionov Y., Li Y., Sawai H., Reed J. C., Perucho M. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997 Feb 14;275(5302):967–969. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- Ropponen K. M., Eskelinen M. J., Lipponen P. K., Alhava E., Kosma V. M. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol. 1997 Jul;182(3):318–324. doi: 10.1002/(SICI)1096-9896(199707)182:3<318::AID-PATH862>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Shi S. R., Key M. E., Kalra K. L. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991 Jun;39(6):741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- Simms L. A., Radford-Smith G., Biden K. G., Buttenshaw R., Cummings M., Jass J. R., Young J., Meltzer S. J., Leggett B. A. Reciprocal relationship between the tumor suppressors p53 and BAX in primary colorectal cancers. Oncogene. 1998 Oct 15;17(15):2003–2008. doi: 10.1038/sj.onc.1202109. [DOI] [PubMed] [Google Scholar]
- Sinicrope F. A., Hart J., Hsu H. A., Lemoine M., Michelassi F., Stephens L. C. Apoptotic and mitotic indices predict survival rates in lymph node-negative colon carcinomas. Clin Cancer Res. 1999 Jul;5(7):1793–1804. [PubMed] [Google Scholar]
- Souza R. F., Appel R., Yin J., Wang S., Smolinski K. N., Abraham J. M., Zou T. T., Shi Y. Q., Lei J., Cottrell J. Microsatellite instability in the insulin-like growth factor II receptor gene in gastrointestinal tumours. Nat Genet. 1996 Nov;14(3):255–257. doi: 10.1038/ng1196-255. [DOI] [PubMed] [Google Scholar]
- Thibodeau S. N., Bren G., Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993 May 7;260(5109):816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- Thibodeau S. N., French A. J., Cunningham J. M., Tester D., Burgart L. J., Roche P. C., McDonnell S. K., Schaid D. J., Vockley C. W., Michels V. V. Microsatellite instability in colorectal cancer: different mutator phenotypes and the principal involvement of hMLH1. Cancer Res. 1998 Apr 15;58(8):1713–1718. [PubMed] [Google Scholar]
- Wright C. M., Dent O. F., Barker M., Newland R. C., Chapuis P. H., Bokey E. L., Young J. P., Leggett B. A., Jass J. R., Macdonald G. A. Prognostic significance of extensive microsatellite instability in sporadic clinicopathological stage C colorectal cancer. Br J Surg. 2000 Sep;87(9):1197–1202. doi: 10.1046/j.1365-2168.2000.01508.x. [DOI] [PubMed] [Google Scholar]