Abstract
BACKGROUND—Villus atrophy is the most distinctive sign of untreated coeliac disease (CD) and epithelial apoptosis is considered to be involved in this stage of the coeliac lesion. The extent of villus atrophy is, however, not homogeneous and patients with patchy or mild lesions have been described. AIMS—To address: (a) the degree of "patchiness" in untreated CD patients; and (b) to clarify if apoptosis, and eventually which trigger drives it, causes epithelial damage. PATIENTS—Twenty of 40 untreated, 14 treated coeliac patients, and 15 controls received five or more multiple duodenal biopsies; the remaining 20 untreated CD patients had no more than three biopsies. METHODS—All biopsies were analysed to monitor the presence of a "flat" mucosa. Biopsies of 14 untreated, 10 treated coeliacs, and seven controls were cultured with or without gliadin. DNA fragmentation was studied by terminal deoxynucleotidyl transferase (TdT) mediated dUTP digoxigenin nick end labelling (TUNEL), and FAS and Ki67 expression by immunohistochemistry. Antiendomysium antibodies (EMA) were surveyed in biopsy culture supernatants. RESULTS—A pattern of patchy duodenal lesions was observed in all untreated CD patients biopsied up to five times. High enterocyte FAS expression, and a high number of TUNEL+ and Ki67+ enterocytes were detected in areas with villus atrophy but not in those with a normal morphology (p<0.001). Conversely, EMA in culture supernatants and signs of immunological activation were present in all untreated CD biopsies. In vitro gliadin challenge increased the number of TUNEL+ and Ki67+ enterocytes (p<0.001 v cultures with medium alone) only in "flat" biopsies. Neutralising anti-FAS monoclonal antibodies were found to control gliadin induced enterocyte apoptosis (p>0.01) while agonist anti-FAS monoclonal antibody increased it (p<0.001). CONCLUSIONS—Patchy lesions are observed in untreated CD mucosa and epithelial FAS engagement is a key trigger in driving villus atrophy in CD. Keywords: apoptosis; FAS; enterocytes; coeliac disease
Full Text
The Full Text of this article is available as a PDF (227.7 KB).
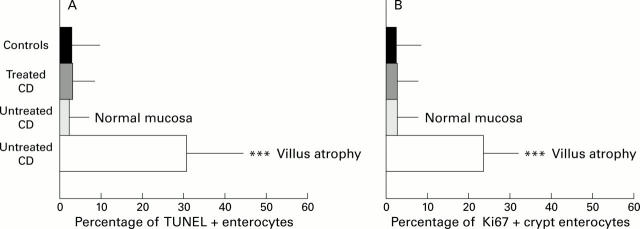
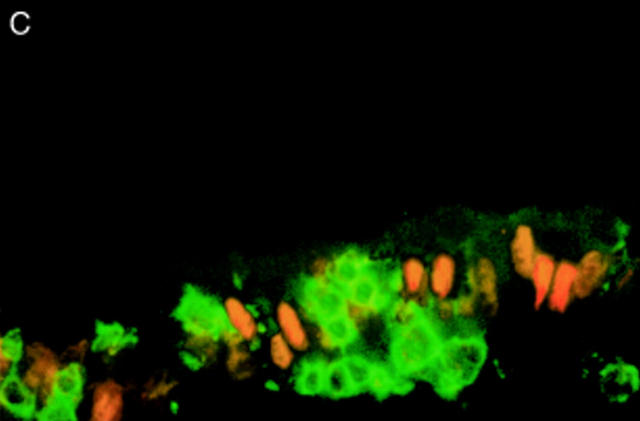
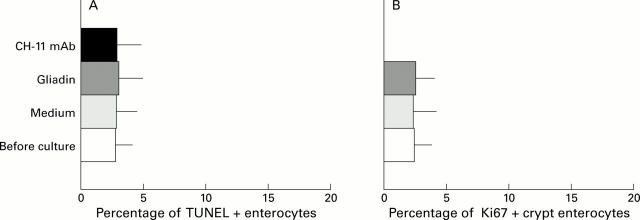
Figure 1 .
DNA fragmentation and Ki67 expression in coeliac disease (CD) and control intestine. (A) DNA fragmentation in enterocytes of CD and control intestine. (B) Expression of Ki67 in crypt enterocytes of CD and control intestine (mean (SD)). Untreated CD, untreated biopsies with patchy mucosal lesions (n=20); treated CD, treated biopsies (n=14); controls, biopsies from non-coeliac patients with a normal villus architecture (n=15). ***p<0.0001 v treated CD and controls. (C) Pattern of intraepithelial DNA fragmentation in untreated CD duodenum. DNA fragmentation (TUNEL technique, red colour) is absent in the nucleus of all intraepithelial lymphocytes (CD3, green colour), while it is detected in the majority of enterocytes. Original magnification ×240. Two colour immunofluorescence: TUNEL technique, RPE labelling (red); immunohistochemistry, CD3 labelling (green) (see methods).
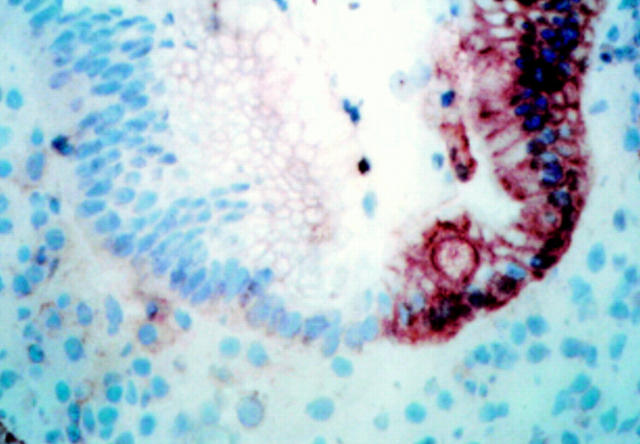
Figure 2 .
FAS expression in untreated coeliac disease (CD) duodenum. A marked change in expression of FAS is observed at the border between regions with normal villus architecture (low expression; left side) and those with villus atrophy (very high expression; right side). Original magnification ×150; immunohistochemistry, peroxidase staining technique.
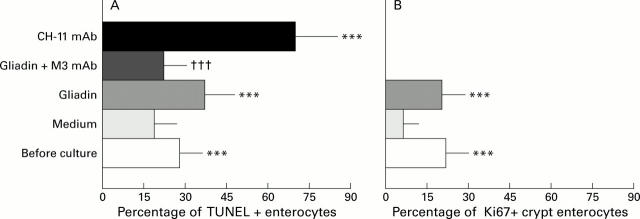
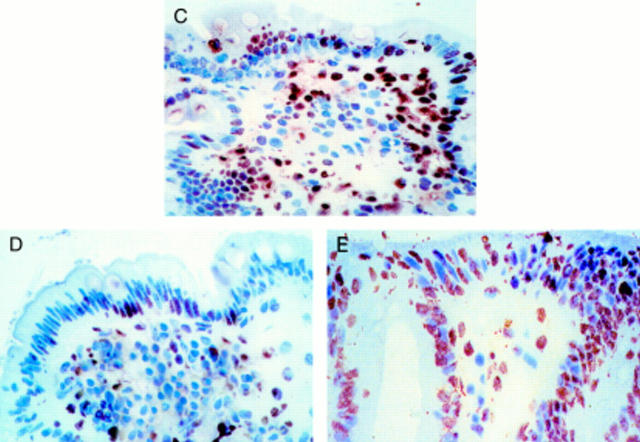
Figure 3 .
In vitro organ culture of untreated coeliac disease (CD) intestine with villus atrophy: DNA fragmentation and Ki67 expression after 24 hour challenge with medium or gliadin. Effect of neutralising anti-FAS M3 or agonist anti-FAS CH-11 monoclonal antibodies (mAbs) on enterocyte apoptosis. (A) DNA fragmentation and (B) Ki67 expression before and after in vitro culture. Untreated CD samples with villus atrophy before in vitro culture (n=7) and after 24 hour challenge with medium alone (n=7), gliadin (n=7), gliadin supplemented with anti-FAS M3 mAb (n=7), or medium supplemented with anti-FAS CH-11 mAb (n=4). The number of TUNEL+ enterocytes (mean (SD)) are given as percentage of both surface and crypt enterocytes and Ki67+ enterocytes (mean (SD)) as percentage of crypt enterocytes. ***p<0.001 v samples after 24 hour challenge with medium alone; †††p=0.001 v samples after 24 hour gliadin challenge. (C) Pattern of DNA fragmentation after 24 hour gliadin challenge. Note that the majority of enterocytes are TUNEL+; staining is also evident in many lamina propria mononuclear cells. (D) Pattern of DNA fragmentation after 24 hours of in vitro challenge with gliadin supplemented with neutralising anti-FAS M3 mAb. The number of TUNEL+ enterocytes is decreased compared with the pattern observed after gliadin challenge. (E) Pattern of DNA fragmentation after 24 hours of in vitro challenge with medium supplemented with agonist CH-11 anti-FAS mAb. A marked increase in the number of TUNEL+ enterocytes with derangement of epithelial architecture is evident. Original magnification: C, D and E (×180); TUNEL technique, peroxidase staining.
Figure 4 .
In vitro organ culture of untreated coeliac disease (CD) intestine with normal villus architecture. DNA fragmentation (A) and Ki67 expression (B) (mean (SD)) after 24 hour challenge with medium or gliadin and effect of agonist anti-FAS CH-11 mAb on enterocyte apoptosis
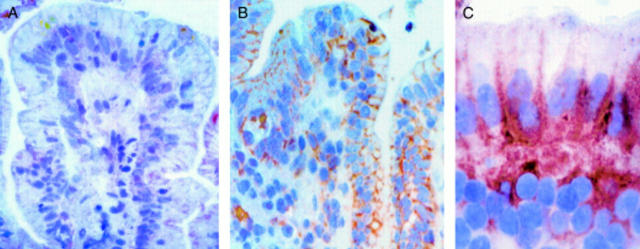
Figure 5 .
In vitro organ culture of treated coeliac disease (CD) intestine: effect of 24 hour gliadin challenge on enterocyte expression of FAS. (A) FAS expression after 24 hour challenge with medium alone. Faint staining is observed on cell membranes of some enterocytes. (B) FAS expression after 24 hour challenge with gliadin. Intense FAS expression is evident on the cell membranes of the majority of enterocytes and in some lamina propria mononuclear cells. (C) FAS expression after 24 hour challenge with gliadin: high magnification. Staining is evident on the basolateral membranes of the enterocytes. Original magnification: A, B (×160); C (×240); immunohistochemistry, peroxidase staining technique.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderson M. R., Tough T. W., Braddy S., Davis-Smith T., Roux E., Schooley K., Miller R. E., Lynch D. H. Regulation of apoptosis and T cell activation by Fas-specific mAb. Int Immunol. 1994 Nov;6(11):1799–1806. doi: 10.1093/intimm/6.11.1799. [DOI] [PubMed] [Google Scholar]
- Auricchio S., De Ritis G., De Vincenzi M., Gentile V., Maiuri L., Mancini E., Porta R., Raia V. Amines protect in vitro the celiac small intestine from the damaging activity of gliadin peptides. Gastroenterology. 1990 Dec;99(6):1668–1674. doi: 10.1016/0016-5085(90)90473-e. [DOI] [PubMed] [Google Scholar]
- Auricchio S., De Ritis G., De Vincenzi M., Magazzù G., Maiuri L., Mancini E., Minetti M., Sapora O., Silano V. Mannan and oligomers of N-acetylglucosamine protect intestinal mucosa of celiac patients with active disease from in vitro toxicity of gliadin peptides. Gastroenterology. 1990 Oct;99(4):973–978. doi: 10.1016/0016-5085(90)90615-8. [DOI] [PubMed] [Google Scholar]
- Biagi F., Parnell N. D., Ellis H. J., Ciclitira P. J. Endomysial antibody production is not related to histological damage after in vitro gluten challenge. Eur J Gastroenterol Hepatol. 2000 Jan;12(1):57–60. doi: 10.1097/00042737-200012010-00011. [DOI] [PubMed] [Google Scholar]
- Kuitunen P., Kosnai I., Savilahti E. Morphometric study of the jejunal mucosa in various childhood enteropathies with special reference to intraepithelial lymphocytes. J Pediatr Gastroenterol Nutr. 1982;1(4):525–531. doi: 10.1097/00005176-198212000-00012. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Wallace P. M., Johnson J., Gibson M. G., Greene J. L., Ledbetter J. A., Singh C., Tepper M. A. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992 Aug 7;257(5071):792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- Loft D. E., Nwokolo C. U., Ciclitira P. J. The diagnosis of gluten sensitivity and coeliac disease--the two are not mutually inclusive. Eur J Gastroenterol Hepatol. 1998 Nov;10(11):911–913. doi: 10.1097/00042737-199811000-00002. [DOI] [PubMed] [Google Scholar]
- MacDonald T. T. Epithelial proliferation in response to gastrointestinal inflammation. Ann N Y Acad Sci. 1992;664:202–209. doi: 10.1111/j.1749-6632.1992.tb39761.x. [DOI] [PubMed] [Google Scholar]
- Maiuri L., Auricchio S., Coletta S., De Marco G., Picarelli A., Di Tola M., Quaratino S., Londei M. Blockage of T-cell costimulation inhibits T-cell action in celiac disease. Gastroenterology. 1998 Sep;115(3):564–572. doi: 10.1016/s0016-5085(98)70135-0. [DOI] [PubMed] [Google Scholar]
- Maiuri L., Picarelli A., Boirivant M., Coletta S., Mazzilli M. C., De Vincenzi M., Londei M., Auricchio S. Definition of the initial immunologic modifications upon in vitro gliadin challenge in the small intestine of celiac patients. Gastroenterology. 1996 May;110(5):1368–1378. doi: 10.1053/gast.1996.v110.pm8613040. [DOI] [PubMed] [Google Scholar]
- Maiuri L., Raia V., De Marco G., Coletta S., de Ritis G., Londei M., Auricchio S. DNA fragmentation is a feature of cystic fibrosis epithelial cells: a disease with inappropriate apoptosis? FEBS Lett. 1997 May 19;408(2):225–231. doi: 10.1016/s0014-5793(97)00347-5. [DOI] [PubMed] [Google Scholar]
- Maiuri L., Rossi M., Raia V., Garipoli V., Hughes L. A., Swallow D., Norén O., Sjöström H., Auricchio S. Mosaic regulation of lactase in human adult-type hypolactasia. Gastroenterology. 1994 Jul;107(1):54–60. doi: 10.1016/0016-5085(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Marsh M. N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue'). Gastroenterology. 1992 Jan;102(1):330–354. [PubMed] [Google Scholar]
- Moss S. F., Attia L., Scholes J. V., Walters J. R., Holt P. R. Increased small intestinal apoptosis in coeliac disease. Gut. 1996 Dec;39(6):811–817. doi: 10.1136/gut.39.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S., Golstein P. The Fas death factor. Science. 1995 Mar 10;267(5203):1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- Picarelli A., Maiuri L., Frate A., Greco M., Auricchio S., Londei M. Production of antiendomysial antibodies after in-vitro gliadin challenge of small intestine biopsy samples from patients with coeliac disease. Lancet. 1996 Oct 19;348(9034):1065–1067. doi: 10.1016/S0140-6736(96)03060-7. [DOI] [PubMed] [Google Scholar]
- Picarelli A., Maiuri L., Mazzilli M. C., Coletta S., Ferrante P., Di Giovambattista F., Greco M., Torsoli A., Auricchio S. Gluten-sensitive disease with mild enteropathy. Gastroenterology. 1996 Sep;111(3):608–616. doi: 10.1053/gast.1996.v111.pm8780564. [DOI] [PubMed] [Google Scholar]
- Przemioslo R. T., Lundin K. E., Sollid L. M., Nelufer J., Ciclitira P. J. Histological changes in small bowel mucosa induced by gliadin sensitive T lymphocytes can be blocked by anti-interferon gamma antibody. Gut. 1995 Jun;36(6):874–879. doi: 10.1136/gut.36.6.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savidge T. C., Shmakov A. N., Walker-Smith J. A., Phillips A. D. Epithelial cell proliferation in childhood enteropathies. Gut. 1996 Aug;39(2):185–193. doi: 10.1136/gut.39.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollid L. M., Thorsby E. HLA susceptibility genes in celiac disease: genetic mapping and role in pathogenesis. Gastroenterology. 1993 Sep;105(3):910–922. doi: 10.1016/0016-5085(93)90912-v. [DOI] [PubMed] [Google Scholar]
- Viard I., Wehrli P., Bullani R., Schneider P., Holler N., Salomon D., Hunziker T., Saurat J. H., Tschopp J., French L. E. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science. 1998 Oct 16;282(5388):490–493. doi: 10.1126/science.282.5388.490. [DOI] [PubMed] [Google Scholar]
- Vogelsang H., Schwarzenhofer M., Granditsch G., Oberhuber G. In vitro production of endomysial antibodies in cultured duodenal mucosa from patients with celiac disease. Am J Gastroenterol. 1999 Apr;94(4):1057–1061. doi: 10.1111/j.1572-0241.1999.01014.x. [DOI] [PubMed] [Google Scholar]