Abstract
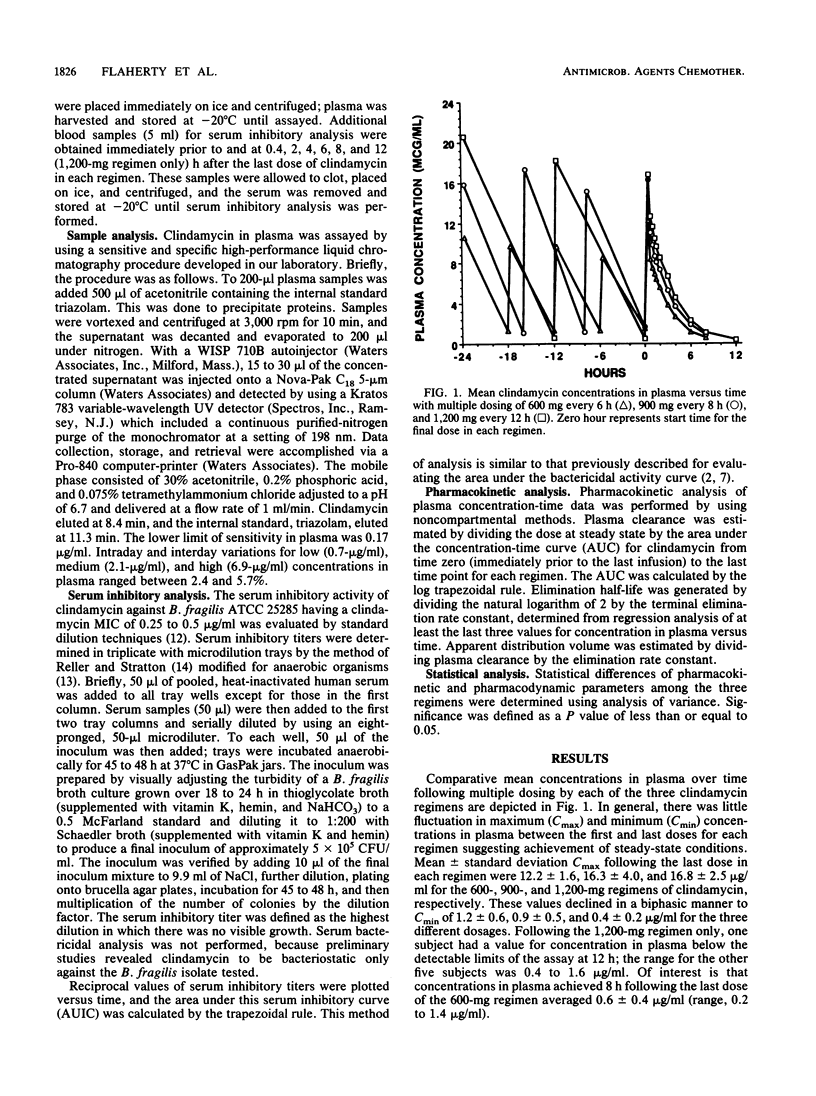
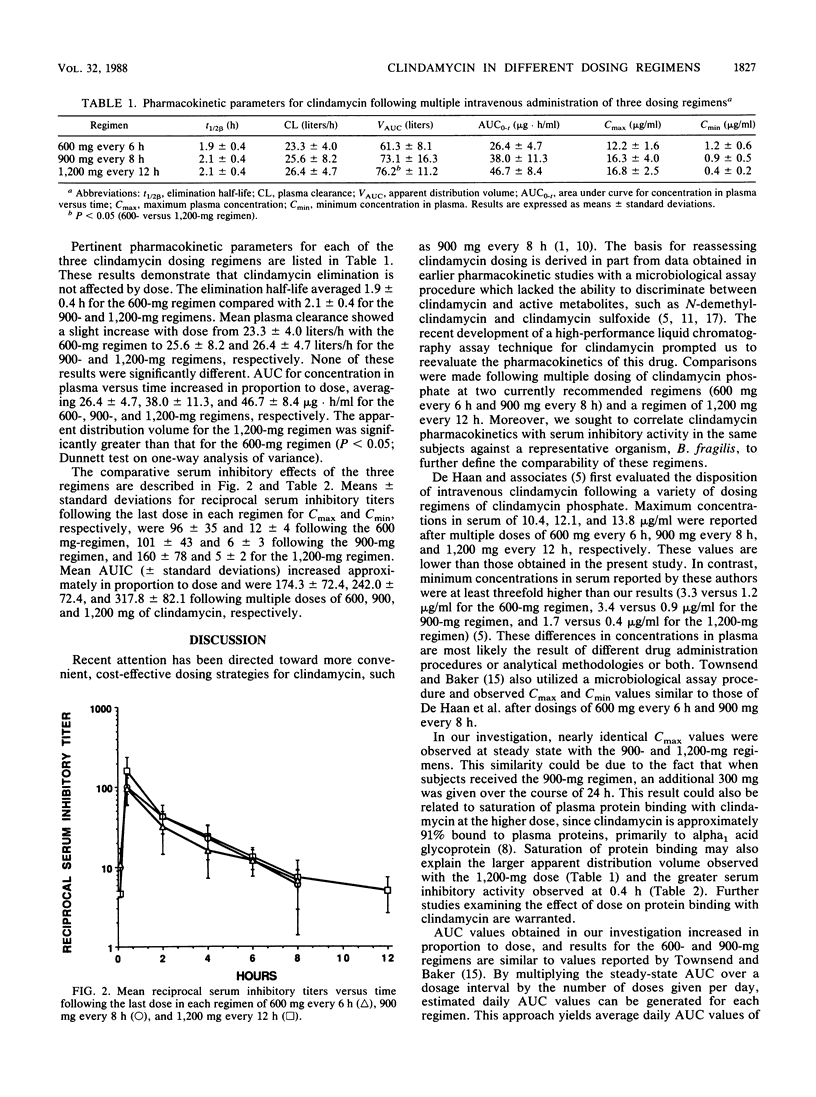
The comparative pharmacokinetics and serum inhibitory effects of clindamycin were evaluated in six healthy male subjects given multiple-dose infusions of the following regimens in a crossover fashion: 600 mg every 6 h, 900 mg every 8 h, and 1,200 mg every 12 h. Serial blood samples were obtained after the last dose in each regimen and analyzed for clindamycin by a sensitive and specific high-performance liquid chromatography assay technique. Clindamycin pharmacokinetics were estimated by using noncompartmental methods, and serum inhibitory titers were serially determined against Bacteroides fragilis ATCC 25285 and evaluated by using area under the serum inhibitory curve (AUIC). Maximum and minimum concentrations in plasma averaged 12.2 +/- 1.6 and 1.2 +/- 0.6, 16.3 +/- 4.0 and 0.9 +/- 0.5, and 16.8 +/- 2.5 and 0.4 +/- 0.2 micrograms/ml for the 600-, 900-, and 1,200-mg regimens, respectively. Clindamycin plasma clearance and elimination half-life averaged 23.3 +/- 4.0 liters/h and 1.9 +/- 0.4 h for the 600-mg regimen, 25.6 +/- 8.2 liters/h and 2.1 +/- 0.4 h for the 900-mg regimen, and 26.4 +/- 4.7 liters/h and 2.1 +/- 0.4 h for the 1,200-mg regimen. These results were not significantly different. Apparent volume of distribution increased significantly for the 1,200-mg regimen compared with the 600-mg regimen. Mean maximum reciprocal serum inhibitory titers were 96 +/- 35, 101 +/- 43, and 160 +/- 78 for the 600-, 900-, and 1,200-mg regimens, respectively. Minimum reciprocal serum inhibitory titers averaged 12 +/- 4, 6 +/- 3, and 5 +/- 2 for the low-, medium-, and high-dose regimens, respectively. Mean AUIC increased roughly in proportion to dose. Similar daily values for the area under the concentration-time curve and for AUIC for each of the regimens suggest similar daily drug exposure and serum inhibitory activity. A regimen of 1,200 mg every 12 h may represent an alternative dosing strategy for clindamycin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel S. R., Olson S. M. Cost analysis of mixing clindamycin and aminoglycosides. Am J Hosp Pharm. 1987 Jun;44(6):1385–1387. [PubMed] [Google Scholar]
- Barriere S. L., Ely E., Kapusnik J. E., Gambertoglio J. G. Analysis of a new method for assessing activity of combinations of antimicrobials: area under the bactericidal activity curve. J Antimicrob Chemother. 1985 Jul;16(1):49–59. doi: 10.1093/jac/16.1.49. [DOI] [PubMed] [Google Scholar]
- Cuchural G. J., Jr, Tally F. P. Bacteroides fragilis: current susceptibilities, mechanisms of drug resistance, and principles of antimicrobial therapy. Drug Intell Clin Pharm. 1986 Jul-Aug;20(7-8):567–573. doi: 10.1177/106002808602000712. [DOI] [PubMed] [Google Scholar]
- Cuchural G. J., Jr, Tally F. P., Jacobus N. V., Aldridge K., Cleary T., Finegold S. M., Hill G., Iannini P., O'Keefe J. P., Pierson C. Susceptibility of the Bacteroides fragilis group in the United States: analysis by site of isolation. Antimicrob Agents Chemother. 1988 May;32(5):717–722. doi: 10.1128/aac.32.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHaan R. M., Metzler C. M., Schellenberg D., Vandenbosch W. D. Pharmacokinetic studies of clindamycin phosphate. J Clin Pharmacol. 1973 May-Jun;13(5):190–209. doi: 10.1002/j.1552-4604.1973.tb00208.x. [DOI] [PubMed] [Google Scholar]
- Drusano G. L. Role of pharmacokinetics in the outcome of infections. Antimicrob Agents Chemother. 1988 Mar;32(3):289–297. doi: 10.1128/aac.32.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty J. F., Barriere S. L., Mordenti J., Gambertoglio J. G. Effect of dose on pharmacokinetics and serum bactericidal activity of mezlocillin. Antimicrob Agents Chemother. 1987 Jun;31(6):895–898. doi: 10.1128/aac.31.6.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R. C., Regamey C., Kirby W. M. Serum protein binding of erythromycin, lincomycin, and clindamycin. J Pharm Sci. 1973 Jul;62(7):1074–1077. doi: 10.1002/jps.2600620704. [DOI] [PubMed] [Google Scholar]
- Harding G. K., Buckwold F. J., Ronald A. R., Marrie T. J., Brunton S., Koss J. C., Gurwith M. J., Albritton W. L. Prospective, randomized comparative study of clindamycin, chloramphenicol, and ticarcillin, each in combination with gentamicin, in therapy for intraabdominal and female genital tract sepsis. J Infect Dis. 1980 Sep;142(3):384–393. doi: 10.1093/infdis/142.3.384. [DOI] [PubMed] [Google Scholar]
- Mansur J. M., Abramowitz P. W., Lerner S. A., Smith R. B., Townsend R. J. Stability and cost analysis of clindamycin-gentamicin admixtures given every eight hours. Am J Hosp Pharm. 1985 Feb;42(2):332–335. [PubMed] [Google Scholar]
- Reller L. B. Interaction of cefotaxime and desacetylcefotaxime against pathogenic bacteria. Assessment with the serum bactericidal test. Diagn Microbiol Infect Dis. 1984 Jun;2(3 Suppl):55S–61S. [PubMed] [Google Scholar]
- Reller L. B., Stratton C. W. Serum dilution test for bactericidal activity. II. Standardization and correlation with antimicrobial assays and susceptibility tests. J Infect Dis. 1977 Aug;136(2):196–204. doi: 10.1093/infdis/136.2.196. [DOI] [PubMed] [Google Scholar]
- Van der Auwera P., Van Laethem Y., Defresne N., Husson M., Klastersky J. Comparative serum bactericidal activity against test anaerobes in volunteers receiving imipenem, clindamycin, latamoxef and metronidazole. J Antimicrob Chemother. 1987 Feb;19(2):205–210. doi: 10.1093/jac/19.2.205. [DOI] [PubMed] [Google Scholar]
- Wagner J. G., Novak E., Patel N. C., Chidester C. G., Lummis W. L. Absorption, excretion and half-life of clinimycin in normal adult males. Am J Med Sci. 1968 Jul;256(1):25–37. doi: 10.1097/00000441-196807000-00004. [DOI] [PubMed] [Google Scholar]


