Abstract
OBJECTIVE—To examine the effects of substance P (endothelium dependent vasodilator) and glyceryl trinitrate (endothelium independent vasodilator) on epicardial coronary arteries in patients with normal coronary angiograms and patients with coronary artery disease. DESIGN—Intracoronary infusions of normal saline, the receptor mediated nitric oxide stimulant substance P (5.6 and 27.8 pmol/min each for five minutes), and glyceryl trinitrate (250 µg bolus) were given in 24 patients with coronary artery disease and stable angina, and in nine patients with normal angiograms. The diameter of proximal and distal coronary segments was measured by computerised quantitative angiography RESULTS—Proximal segments of patients with coronary artery disease dilated less than those of patients with normal angiograms in response to 27.8 pmol/min substance P (mean (SEM): 7.9 (1.3)% v 15 (2.3)% respectively, p < 0.01). The proximal segments of diseased arteries also dilated less than those of "normal" arteries in response to glyceryl trinitrate (10.2 (1.6)% v 18.4 (2.9)%, respectively, p < 0.01). The responses of distal segments to substance P and glyceryl trinitrate were similar in the two patient groups. There were correlations (all p < 0.001) between the coronary diameter after substance P and after glyceryl trinitrate in normal proximal segments (r = 0.94) and normal distal segments (r = 0.64), in diseased proximal segments (r = 0.95) and diseased distal segments (r = 0.89), and for coronary stenoses (r = 0.93). CONCLUSIONS—Proximal segments of patients with coronary disease dilated less than the proximal segments of "normal" patients in response to substance P and glyceryl trinitrate. The response to substance P is substantial and closely correlated with the response to glyceryl trinitrate in both "normal" patients and those with coronary disease. This suggests that although the proximal segments of diseased coronary arteries have a reduced capacity to dilate in response to direct stimulation of smooth muscle cell relaxation, they retain much of their endothelium dependent vasodilator function. Keywords: endothelium; nitric oxide; coronary artery disease; glyceryl trinitrate
Full Text
The Full Text of this article is available as a PDF (118.6 KB).
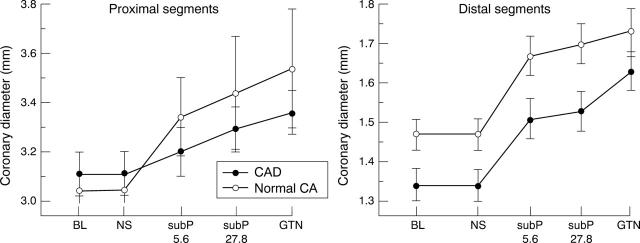
Figure 1 .
Plot showing mean dose dependent responses (mean coronary luminal diameter with SEM) to substance P (subP) and glyceryl trinitrate (GTN) for proximal and distal segments in patients with coronary artery disease (CAD) and in patients with "normal" coronary arteries (CA). A progressive increase in mean luminal diameter occurred with increasing doses of substances P and glyceryl trinitrate administration. BL, baseline; NS, saline.
Figure 2 .
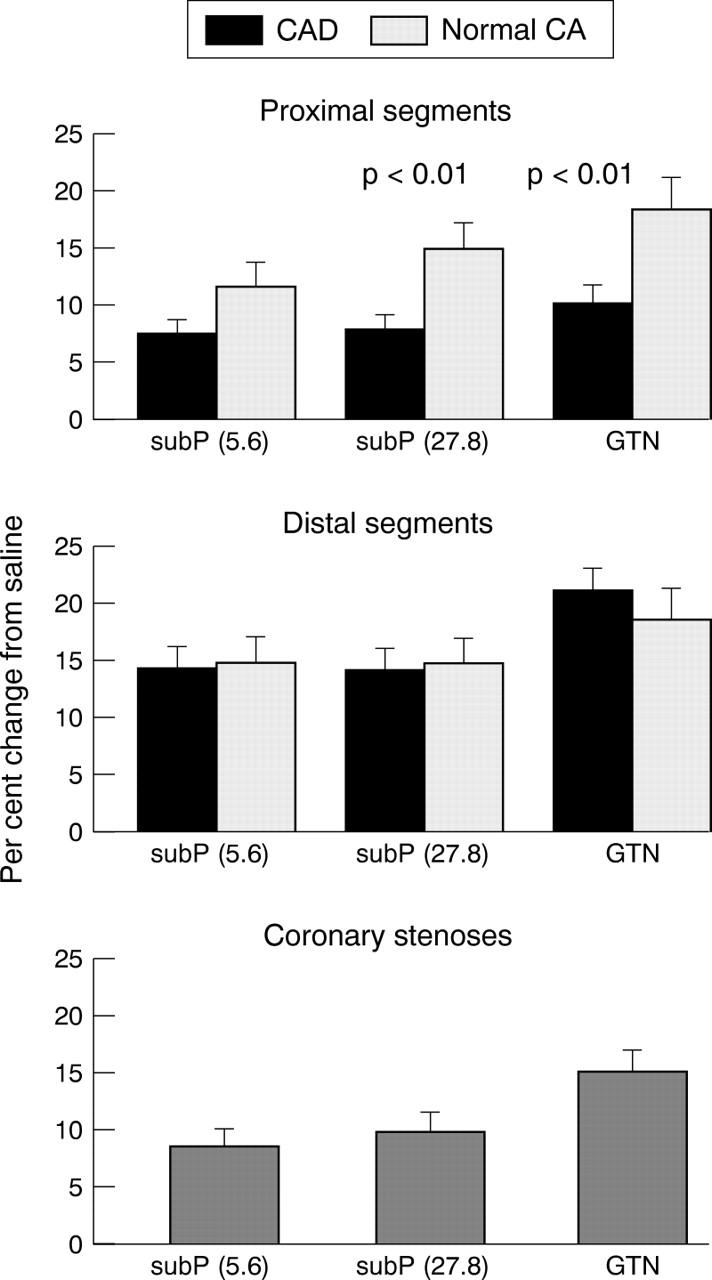
Bar graph showing mean (with SEM) percentage dilatation from saline after intracoronary infusion of 5.6 and 27.8 pmol/min substance P (subP) and after glyceryl trinitrate (GTN) administration for proximal and distal coronary segments in patients with "normal" coronary arteries (CA) and in patients with coronary artery disease (CAD) and in coronary stenoses. In patients with normal coronary arteries, proximal segments showed a significantly greater dilatation (p < 0.01) during substance P and glyceryl trinitrate infusions than in patients with coronary artery disease.
Figure 3 .
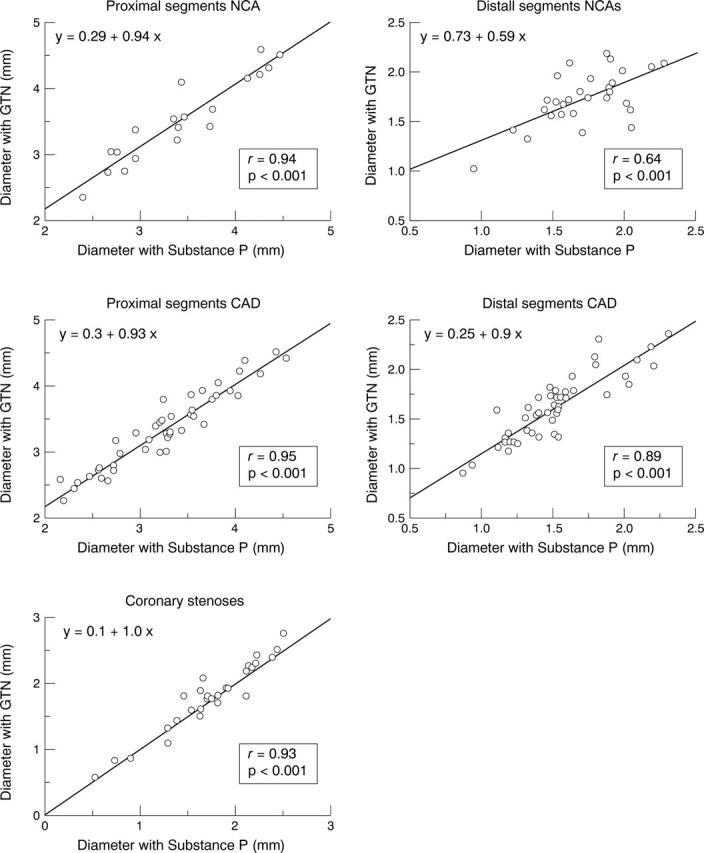
Scatterplot showing the relation between the coronary diameter in response to glyceryl trinitrate (GTN) and to substance P in proximal and distal segments in patients with "normal" coronary angiograms (NCAs) and in patients with coronary artery disease (CAD) and in coronary stenoses. Linear regression analysis show a significant correlation between these two variables in both groups. The slope of this linear relation between these two variables was close to unity (0.9-1.0).
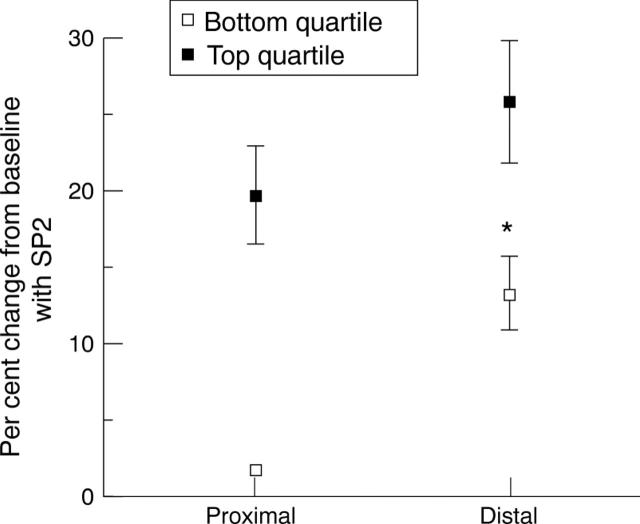
Figure 4 .
Graph showing per cent response to 27.8 pmol/min substance P (SP2) from baseline of the bottom and top quartiles of proximal segments together with the response of their corresponding distal segments in patients with coronary artery disease. The proximal segments of diseased coronary arteries were divided into quartiles according to their response to substance P. The distal segment response to substance P is greater than the proximal segment response in both groups (p < 0.01), but less in the bottom quartile than in the top quartile (*p < 0.05).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulanger C., Hendrickson H., Lorenz R. R., Vanhoutte P. M. Release of different relaxing factors by cultured porcine endothelial cells. Circ Res. 1989 Jun;64(6):1070–1078. doi: 10.1161/01.res.64.6.1070. [DOI] [PubMed] [Google Scholar]
- Brown B. G., Bolson E., Petersen R. B., Pierce C. D., Dodge H. T. The mechanisms of nitroglycerin action: stenosis vasodilatation as a major component of the drug response. Circulation. 1981 Dec;64(6):1089–1097. doi: 10.1161/01.cir.64.6.1089. [DOI] [PubMed] [Google Scholar]
- Bull H. A., Hothersall J., Chowdhury N., Cohen J., Dowd P. M. Neuropeptides induce release of nitric oxide from human dermal microvascular endothelial cells. J Invest Dermatol. 1996 Apr;106(4):655–660. doi: 10.1111/1523-1747.ep12345471. [DOI] [PubMed] [Google Scholar]
- Buttery L. D., Springall D. R., Chester A. H., Evans T. J., Standfield E. N., Parums D. V., Yacoub M. H., Polak J. M. Inducible nitric oxide synthase is present within human atherosclerotic lesions and promotes the formation and activity of peroxynitrite. Lab Invest. 1996 Jul;75(1):77–85. [PubMed] [Google Scholar]
- Chester A. H., O'Neil G. S., Moncada S., Tadjkarimi S., Yacoub M. H. Low basal and stimulated release of nitric oxide in atherosclerotic epicardial coronary arteries. Lancet. 1990 Oct 13;336(8720):897–900. doi: 10.1016/0140-6736(90)92269-n. [DOI] [PubMed] [Google Scholar]
- Christie M. I., Griffith T. M., Lewis M. J. A comparison of basal and agonist-stimulated release of endothelium-derived relaxing factor from different arteries. Br J Pharmacol. 1989 Oct;98(2):397–406. doi: 10.1111/j.1476-5381.1989.tb12610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft J. R., Chowienczyk P. J., Brett S. E., Ritter J. M. Effect of NG-monomethyl-L-arginine on kinin-induced vasodilation in the human forearm. Br J Clin Pharmacol. 1994 Oct;38(4):307–310. doi: 10.1111/j.1365-2125.1994.tb04358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D. A., Vita J. A., Treasure C. B., Fish R. D., Alexander R. W., Ganz P., Selwyn A. P. Atherosclerosis impairs flow-mediated dilation of coronary arteries in humans. Circulation. 1989 Sep;80(3):458–465. doi: 10.1161/01.cir.80.3.458. [DOI] [PubMed] [Google Scholar]
- Crossman D. C., Larkin S. W., Dashwood M. R., Davies G. J., Yacoub M., Maseri A. Responses of atherosclerotic human coronary arteries in vivo to the endothelium-dependent vasodilator substance P. Circulation. 1991 Nov;84(5):2001–2010. doi: 10.1161/01.cir.84.5.2001. [DOI] [PubMed] [Google Scholar]
- Crossman D. C., Larkin S. W., Fuller R. W., Davies G. J., Maseri A. Substance P dilates epicardial coronary arteries and increases coronary blood flow in humans. Circulation. 1989 Sep;80(3):475–484. doi: 10.1161/01.cir.80.3.475. [DOI] [PubMed] [Google Scholar]
- Enokibori M., Okamura T., Toda N. Mechanism underlying substance P-induced relaxation in dog isolated superficial temporal arteries. Br J Pharmacol. 1994 Jan;111(1):77–82. doi: 10.1111/j.1476-5381.1994.tb14026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. L., Marx J. D., Pepine C. J., Conti C. R. Analysis of coronary responses to various doses of intracoronary nitroglycerin. Circulation. 1982 Aug;66(2):321–327. doi: 10.1161/01.cir.66.2.321. [DOI] [PubMed] [Google Scholar]
- Feletou M., Vanhoutte P. M. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol. 1988 Mar;93(3):515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Mügge A., Alheid U., Bode S. M., Frölich J. C. Endothelium-derived relaxing factor (EDRF): a defence mechanism against platelet aggregation and vasospasm in human coronary arteries. Eur Heart J. 1989 Nov;10 (Suppl F):36–43. doi: 10.1093/eurheartj/10.suppl_f.36. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Mügge A., Alheid U., Haverich A., Frölich J. C. Selective attenuation of endothelium-mediated vasodilation in atherosclerotic human coronary arteries. Circ Res. 1988 Feb;62(2):185–190. doi: 10.1161/01.res.62.2.185. [DOI] [PubMed] [Google Scholar]
- Golino P., Piscione F., Willerson J. T., Cappelli-Bigazzi M., Focaccio A., Villari B., Indolfi C., Russolillo E., Condorelli M., Chiariello M. Divergent effects of serotonin on coronary-artery dimensions and blood flow in patients with coronary atherosclerosis and control patients. N Engl J Med. 1991 Mar 7;324(10):641–648. doi: 10.1056/NEJM199103073241001. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Newby A. C., Henderson A. H. The nature of endothelium-derived vascular relaxant factor. Nature. 1984 Apr 12;308(5960):645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- Hutchinson P. J., Palmer R. M., Moncada S. Comparative pharmacology of EDRF and nitric oxide on vascular strips. Eur J Pharmacol. 1987 Sep 23;141(3):445–451. doi: 10.1016/0014-2999(87)90563-2. [DOI] [PubMed] [Google Scholar]
- Kaski J. C., Tousoulis D., Gavrielides S., McFadden E., Galassi A. R., Crea F., Maseri A. Comparison of epicardial coronary artery tone and reactivity in Prinzmetal's variant angina and chronic stable angina pectoris. J Am Coll Cardiol. 1991 Apr;17(5):1058–1062. doi: 10.1016/0735-1097(91)90830-3. [DOI] [PubMed] [Google Scholar]
- Kilpatrick E. V., Cocks T. M. Evidence for differential roles of nitric oxide (NO) and hyperpolarization in endothelium-dependent relaxation of pig isolated coronary artery. Br J Pharmacol. 1994 Jun;112(2):557–565. doi: 10.1111/j.1476-5381.1994.tb13110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroiwa M., Aoki H., Kobayashi S., Nishimura J., Kanaide H. Mechanism of endothelium-dependent relaxation induced by substance P in the coronary artery of the pig. Br J Pharmacol. 1995 Oct;116(3):2040–2047. doi: 10.1111/j.1476-5381.1995.tb16409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushwaha S. S., Crossman D. C., Bustami M., Davies G. J., Mitchell A. G., Maseri A., Yacoub M. H. Substance P for evaluation of coronary endothelial function after cardiac transplantation. J Am Coll Cardiol. 1991 Jun;17(7):1537–1544. doi: 10.1016/0735-1097(91)90644-o. [DOI] [PubMed] [Google Scholar]
- Ludmer P. L., Selwyn A. P., Shook T. L., Wayne R. R., Mudge G. H., Alexander R. W., Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986 Oct 23;315(17):1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- Numaguchi K., Egashira K., Takemoto M., Kadokami T., Shimokawa H., Sueishi K., Takeshita A. Chronic inhibition of nitric oxide synthesis causes coronary microvascular remodeling in rats. Hypertension. 1995 Dec;26(6 Pt 1):957–962. doi: 10.1161/01.hyp.26.6.957. [DOI] [PubMed] [Google Scholar]
- Panza J. A., Quyyumi A. A., Brush J. E., Jr, Epstein S. E. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990 Jul 5;323(1):22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- Petersson J., Zygmunt P. M., Brandt L., Högestätt E. D. Substance P-induced relaxation and hyperpolarization in human cerebral arteries. Br J Pharmacol. 1995 Jul;115(6):889–894. doi: 10.1111/j.1476-5381.1995.tb15893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quyyumi A. A., Mulcahy D., Andrews N. P., Husain S., Panza J. A., Cannon R. O., 3rd Coronary vascular nitric oxide activity in hypertension and hypercholesterolemia. Comparison of acetylcholine and substance P. Circulation. 1997 Jan 7;95(1):104–110. doi: 10.1161/01.cir.95.1.104. [DOI] [PubMed] [Google Scholar]
- Ralevic V., Khalil Z., Helme R. D., Dusting G. J. Role of nitric oxide in the actions of substance P and other mediators of inflammation in rat skin microvasculature. Eur J Pharmacol. 1995 Sep 25;284(3):231–239. doi: 10.1016/0014-2999(95)00321-b. [DOI] [PubMed] [Google Scholar]
- Reiber J. H., Serruys P. W., Kooijman C. J., Wijns W., Slager C. J., Gerbrands J. J., Schuurbiers J. C., den Boer A., Hugenholtz P. G. Assessment of short-, medium-, and long-term variations in arterial dimensions from computer-assisted quantitation of coronary cineangiograms. Circulation. 1985 Feb;71(2):280–288. doi: 10.1161/01.cir.71.2.280. [DOI] [PubMed] [Google Scholar]
- Schächinger V., Zeiher A. M. Quantitative assessment of coronary vasoreactivity in humans in vivo. Importance of baseline vasomotor tone in atherosclerosis. Circulation. 1995 Oct 15;92(8):2087–2094. doi: 10.1161/01.cir.92.8.2087. [DOI] [PubMed] [Google Scholar]
- Shikano K., Ohlstein E. H., Berkowitz B. A. Differential selectivity of endothelium-derived relaxing factor and nitric oxide in smooth muscle. Br J Pharmacol. 1987 Nov;92(3):483–485. doi: 10.1111/j.1476-5381.1987.tb11347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousoulis D., Crake T., Davies G. Basal nitric oxide production by diseased coronary arteries. J Am Coll Cardiol. 1996 Nov 15;28(6):1639–1640. doi: 10.1016/s0735-1097(96)90210-7. [DOI] [PubMed] [Google Scholar]
- Tousoulis D., Davies G. J., Tentolouris C., Crake T., Lefroy D. C., Toutouzas P. Effects of inhibition of nitric oxide synthesis in patients with coronary artery disease and stable angina. Eur Heart J. 1997 Apr;18(4):608–613. doi: 10.1093/oxfordjournals.eurheartj.a015304. [DOI] [PubMed] [Google Scholar]
- Tousoulis D., Davies G., McFadden E., Clarke J., Kaski J. C., Maseri A. Coronary vasomotor effects of serotonin in patients with angina. Relation to coronary stenosis morphology. Circulation. 1993 Oct;88(4 Pt 1):1518–1526. doi: 10.1161/01.cir.88.4.1518. [DOI] [PubMed] [Google Scholar]
- Tousoulis D., Kaski J. C., Bogaty P., Crea F., Gavrielides S., Galassi A. R., Maseri A. Reactivity of proximal and distal angiographically normal and stenotic coronary segments in chronic stable angina pectoris. Am J Cardiol. 1991 Jun 1;67(15):1195–1200. doi: 10.1016/0002-9149(91)90926-c. [DOI] [PubMed] [Google Scholar]
- Tousoulis D., Tentolouris C., Crake T., Toutouzas P., Davies G. Basal and flow-mediated nitric oxide production by atheromatous coronary arteries. J Am Coll Cardiol. 1997 May;29(6):1256–1262. doi: 10.1016/s0735-1097(97)00046-6. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Auch-Schwelk W., Biondi M. L., Lorenz R. R., Schini V. B., Vidal M. J. Why are converting enzyme inhibitors vasodilators? Br J Clin Pharmacol. 1989;28 (Suppl 2):95S–104S. doi: 10.1111/j.1365-2125.1989.tb03585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte P. M. The endothelium--modulator of vascular smooth-muscle tone. N Engl J Med. 1988 Aug 25;319(8):512–513. doi: 10.1056/NEJM198808253190809. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M. The endothelium--modulator of vascular smooth-muscle tone. N Engl J Med. 1988 Aug 25;319(8):512–513. doi: 10.1056/NEJM198808253190809. [DOI] [PubMed] [Google Scholar]
- Weihe E., Reinecke M., Opherk D., Forssmann W. G. Peptidergic innervation (substance P) in the human heart. J Mol Cell Cardiol. 1981 Mar;13(3):331–333. doi: 10.1016/0022-2828(81)90321-7. [DOI] [PubMed] [Google Scholar]
- Zeiher A. M., Drexler H., Wollschlaeger H., Saurbier B., Just H. Coronary vasomotion in response to sympathetic stimulation in humans: importance of the functional integrity of the endothelium. J Am Coll Cardiol. 1989 Nov 1;14(5):1181–1190. doi: 10.1016/0735-1097(89)90414-2. [DOI] [PubMed] [Google Scholar]
- Zeiher A. M., Drexler H., Wollschläger H., Just H. Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation. 1991 Feb;83(2):391–401. doi: 10.1161/01.cir.83.2.391. [DOI] [PubMed] [Google Scholar]
- Ziche M., Morbidelli L., Masini E., Amerini S., Granger H. J., Maggi C. A., Geppetti P., Ledda F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994 Nov;94(5):2036–2044. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]