Abstract
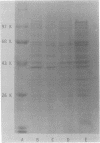
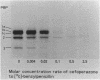
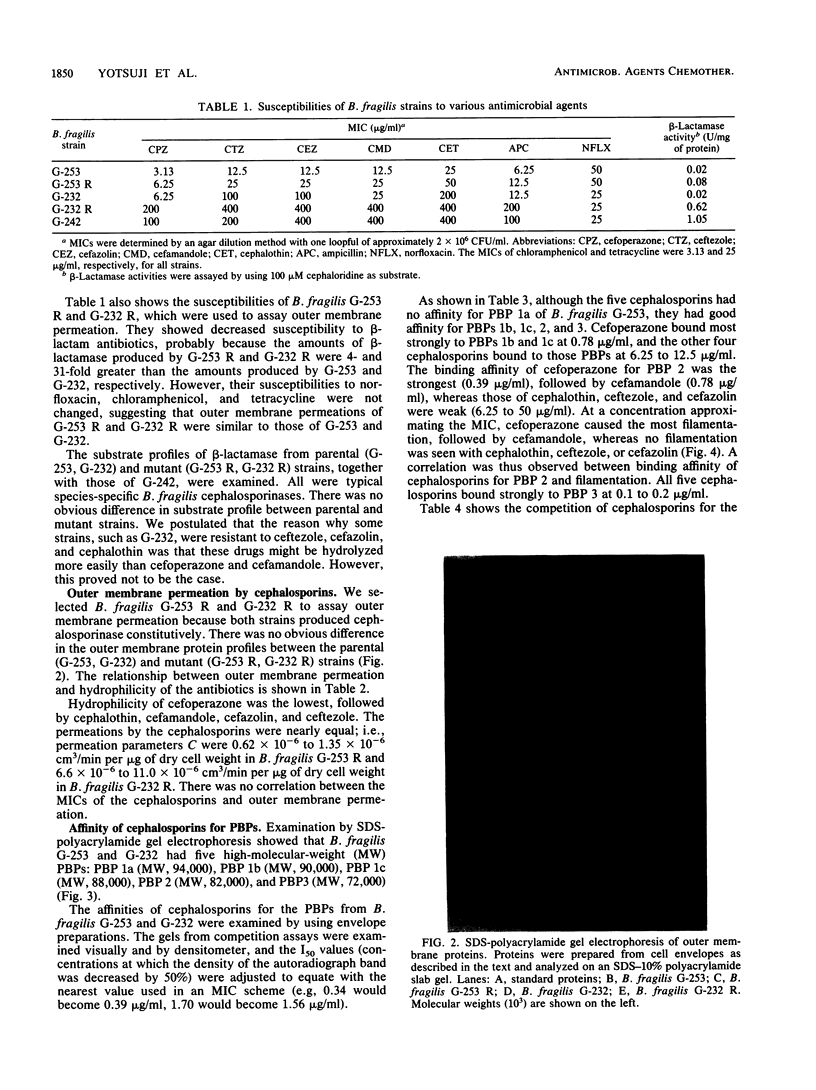
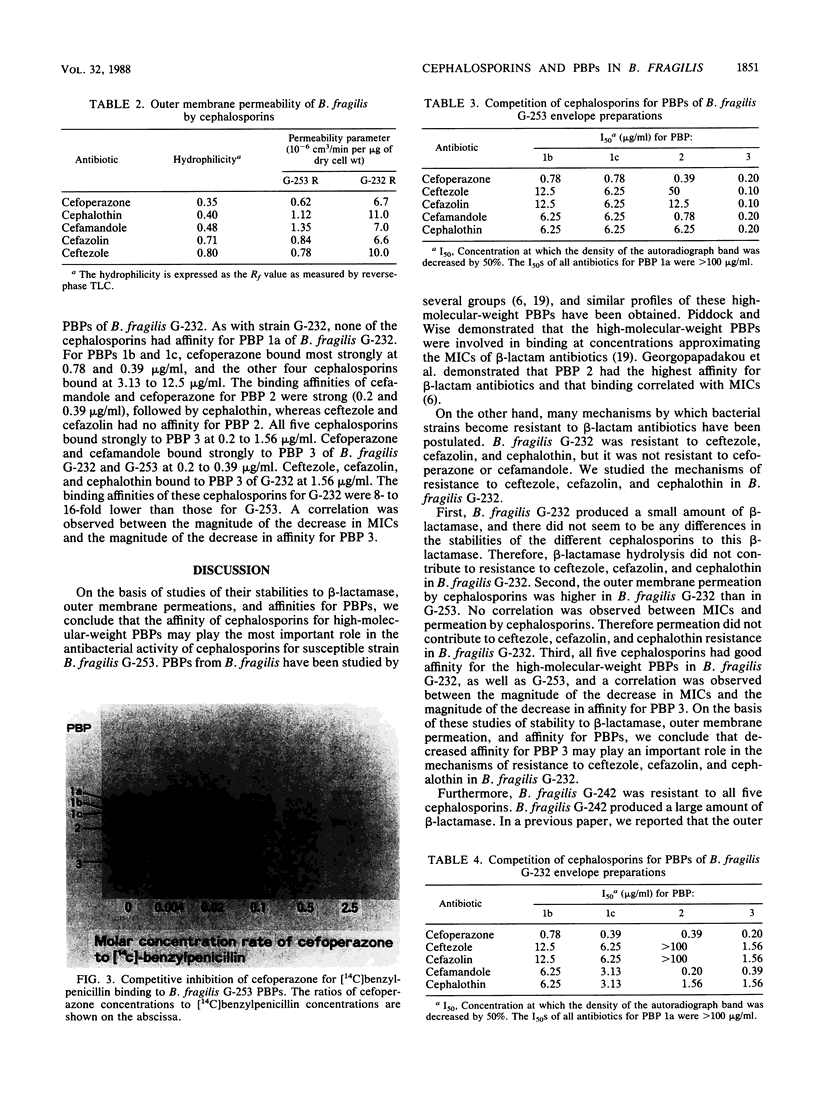
The susceptibilities of 52 clinical isolates of Bacteroides fragilis to five monoanionic cephalosporins were examined. Cefoperazone showed the highest antibacterial activity, followed by ceftezole, cefazolin, cefamandole, and cephalothin. There were two groups of resistant strains: one group (ca. 15%), of which B. fragilis G-232 was a typical sample, was resistant to ceftezole (MIC, 100 micrograms/ml), cefazolin (MIC, 100 micrograms/ml), and cephalothin (MIC, 200 micrograms/ml) but not cefoperazone (MIC, 6.25 micrograms/ml) or cefamandole (MIC, 25 micrograms/ml). On the basis of studies of stability to beta-lactamase, outer membrane permeation, and affinity for penicillin-binding proteins (PBPs), we conclude that decreased affinity for PBP 3 may play an important role in the resistance to ceftezole, cefazolin, and cephalothin in B. fragilis G-232. Another group (also ca. 15%), of which B. fragilis G-242 was a representative, was resistant to all five cephalosporins (MIC, 100 to 400 micrograms/ml) and produced a high amount of beta-lactamase. Similar broad-spectrum resistance was seen in a mutant of strain G-232 that had a greater-than-30-fold increase in beta-lactamase production.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G. Properties of penicillin-binding proteins in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1981 Feb;19(2):316–322. doi: 10.1128/aac.19.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K., Tanaka S. K., Bonner D. P., Sykes R. B. Resistance caused by decreased penetration of beta-lactam antibiotics into Enterobacter cloacae. Antimicrob Agents Chemother. 1985 Apr;27(4):555–560. doi: 10.1128/aac.27.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty T. J., Koller A. E., Tomasz A. Penicillin-binding proteins of penicillin-susceptible and intrinsically resistant Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1980 Nov;18(5):730–737. doi: 10.1128/aac.18.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold S. M. Pathogenic anaerobes. Arch Intern Med. 1982 Oct 25;142(11):1988–1992. [PubMed] [Google Scholar]
- Georgopapadakou N. H., Smith S. A., Sykes R. B. Penicillin-binding proteins in Bacteroides fragilis. J Antibiot (Tokyo) 1983 Jul;36(7):907–910. doi: 10.7164/antibiotics.36.907. [DOI] [PubMed] [Google Scholar]
- Godfrey A. J., Bryan L. E., Rabin H. R. beta-Lactam-resistant Pseudomonas aeruginosa with modified penicillin-binding proteins emerging during cystic fibrosis treatment. Antimicrob Agents Chemother. 1981 May;19(5):705–711. doi: 10.1128/aac.19.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Nuchamowitz Y., Rubinstein E. Insensitivity of peptidoglycan biosynthetic reactions to beta-lactam antibiotics in a clinical isolate of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1981 May;19(5):687–695. doi: 10.1128/aac.19.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyama J., Hiruma R., Yamaguchi A., Sawai T. Identification of porins in outer membrane of Proteus, Morganella, and Providencia spp. and their role in outer membrane permeation of beta-lactams. Antimicrob Agents Chemother. 1987 Mar;31(3):379–384. doi: 10.1128/aac.31.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. Micro-iodometric assay for penicillinase. Biochem J. 1962 May;83:236–240. doi: 10.1042/bj0830236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobles E. R., Jr Bacteroides infections. Ann Surg. 1973 May;177(5):601–606. [PMC free article] [PubMed] [Google Scholar]
- Olsson B., Dornbusch K., Nord C. E. Factors contributing to resistance to beta-lactam antibiotics in Bacteroides fragilis. Antimicrob Agents Chemother. 1979 Feb;15(2):263–268. doi: 10.1128/aac.15.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson B., Nord C. E., Wadström T. Formation of beta-lactamase in Bacteroides fragilis: cell-bound and extracellular activity. Antimicrob Agents Chemother. 1976 May;9(5):727–735. doi: 10.1128/aac.9.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piddock L. J., Wise R. Properties of the penicillin-binding proteins of four species of the genus Bacteroides. Antimicrob Agents Chemother. 1986 May;29(5):825–832. doi: 10.1128/aac.29.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. E., Brown D. F. Penicillin-binding proteins of beta-lactam-resistant strains of Staphylococcus aureus. Effect of growth conditions. FEBS Lett. 1985 Nov 11;192(1):28–32. doi: 10.1016/0014-5793(85)80036-3. [DOI] [PubMed] [Google Scholar]
- Rossi L., Tonin E., Cheng Y. R., Fontana R. Regulation of penicillin-binding protein activity: description of a methicillin-inducible penicillin-binding protein in Staphylococcus aureus. Antimicrob Agents Chemother. 1985 May;27(5):828–831. doi: 10.1128/aac.27.5.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Inoue M., Mitsuhashi S. Activity of beta-lactamase produced by Bacteroides fragilis against newly introduced cephalosporins. Antimicrob Agents Chemother. 1980 Apr;17(4):736–737. doi: 10.1128/aac.17.4.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Matsuba K., Yamagishi S. A method for measuring the outer membrane-permeability of beta-lactam antibiotics in gram-negative bacteria. J Antibiot (Tokyo) 1977 Dec;30(12):1134–1136. doi: 10.7164/antibiotics.30.1134. [DOI] [PubMed] [Google Scholar]
- Simpson I. N., Page C. D., Harper P. B. The contribution of beta-lactamases to beta-lactam resistance in Bacteroides fragilis. J Antimicrob Chemother. 1982 Jan;9(1):29–45. doi: 10.1093/jac/9.1.29. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Timewell R., Taylor E., Phillips I. The beta-lactamases of Bacteroides species. J Antimicrob Chemother. 1981 Feb;7(2):137–146. doi: 10.1093/jac/7.2.137. [DOI] [PubMed] [Google Scholar]
- Werner V., Sanders C. C., Sanders W. E., Jr, Goering R. V. Role of beta-lactamases and outer membrane proteins in multiple beta-lactam resistance of Enterobacter cloacae. Antimicrob Agents Chemother. 1985 Apr;27(4):455–459. doi: 10.1128/aac.27.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A., Tomiyama N., Hiruma R., Sawai T. Difference in pathway of Escherichia coli outer membrane permeation between penicillins and cephalosporins. FEBS Lett. 1985 Feb 11;181(1):143–148. doi: 10.1016/0014-5793(85)81130-3. [DOI] [PubMed] [Google Scholar]
- Yotsuji A., Minami S., Inoue M., Mitsuhashi S. Properties of novel beta-lactamase produced by Bacteroides fragilis. Antimicrob Agents Chemother. 1983 Dec;24(6):925–929. doi: 10.1128/aac.24.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsuji A., Minami S., Kakizawa H., Yasuda T., Takai A., Saikawa I., Inoue M., Mitsuhashi S. Cephamycin inactivation due to enzymatic hydrolysis by beta-lactamase from Bacteroides fragilis. Antimicrob Agents Chemother. 1985 Dec;28(6):773–777. doi: 10.1128/aac.28.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotsuji A., Mitsuyama J., Hori R., Yasuda T., Saikawa I., Inoue M., Mitsuhashi S. Outer membrane permeation of Bacteroides fragilis by cephalosporins. Antimicrob Agents Chemother. 1988 Jul;32(7):1097–1099. doi: 10.1128/aac.32.7.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]