Abstract
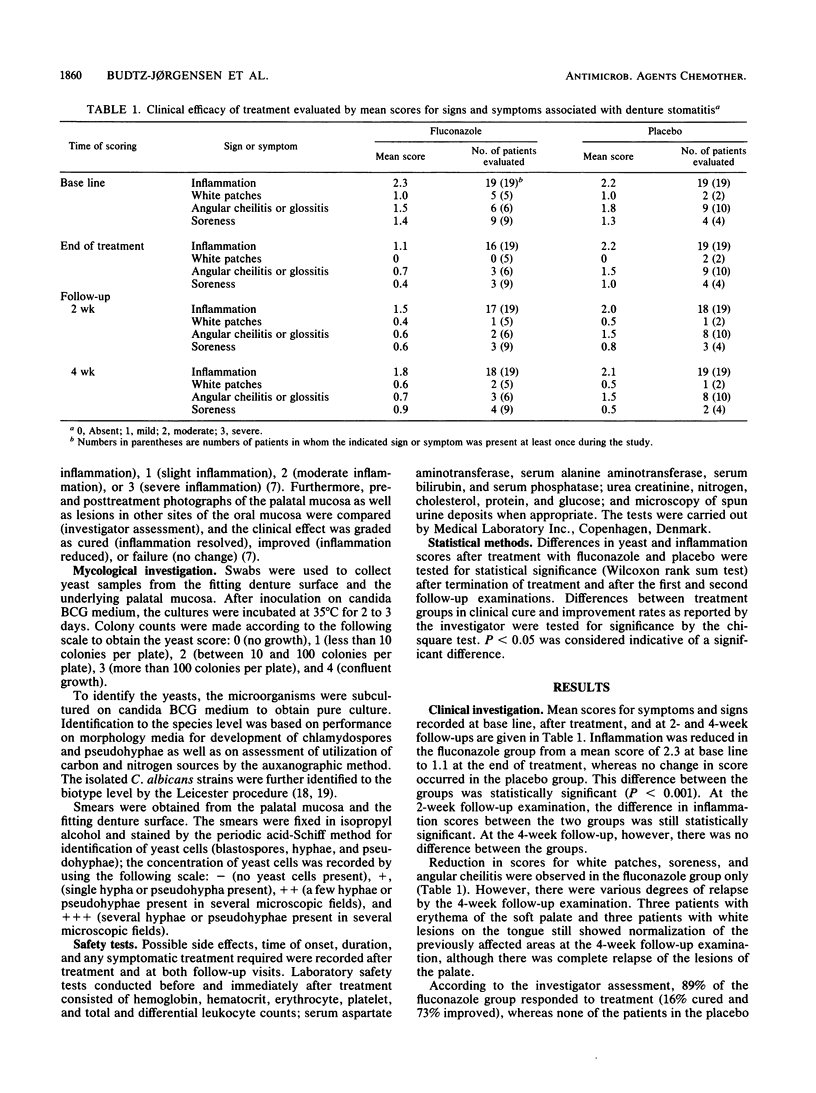
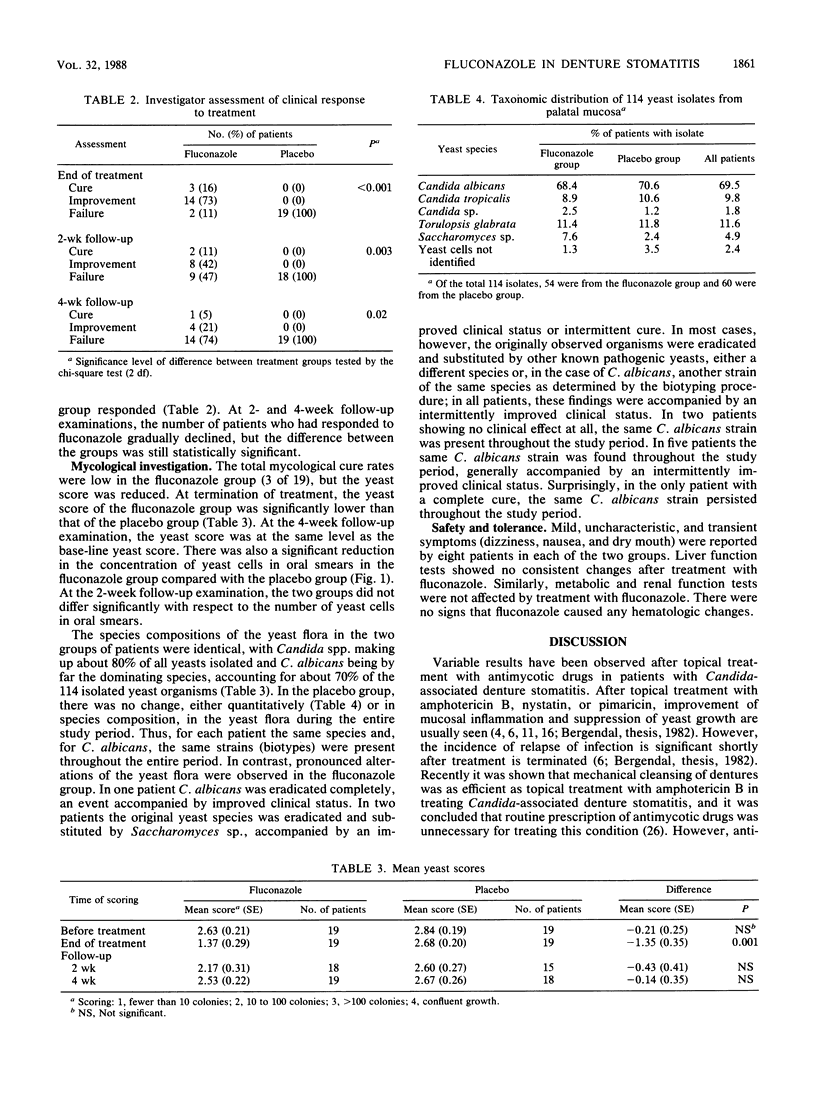
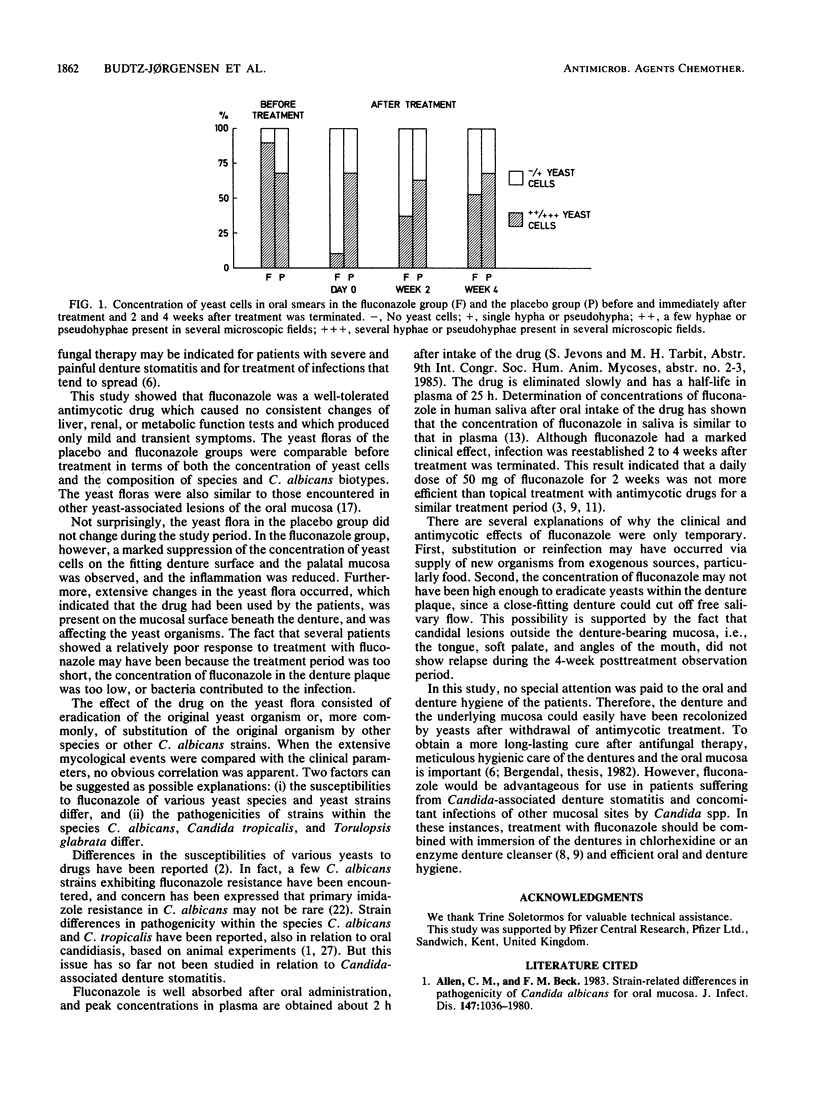
A double-blind trial was carried out to study the effect of oral administration of fluconazole in the treatment of Candida-associated denture stomatitis. The study group consisted of 38 denture stomatitis patients who harbored yeasts, predominantly Candida spp., in significant numbers as determined by culture from the lesions. Half of the patients received 50 mg of fluconazole per day orally for 14 days, and the other half received placebo capsules. The following parameters were studied: degree of palatal erythema, presence of yeast cells (by plate count and microscopy of smears), identification to the species level of dominant yeast organisms, biotyping of Candida albicans, and treatment-related side effects. A significant reduction of erythema was seen after treatment with fluconazole, but the inflammation showed partial relapse 2 to 4 weeks after treatment was terminated. Reduced soreness of the oral mucosa was reported by six of the patients in the fluconazole group. No significant clinical or yeast flora changes were observed in the placebo group. Extensive changes in the yeast flora were observed in the fluconazole group, both in quantity and in composition of yeast species and C. albicans strains (biotypes), which perhaps indicated differences in pathogenicity and fluconazole susceptibility among various yeast species and C. albicans strains. Fluconazole did not produce any changes in the results of blood and urine analyses. The results indicate that fluconazole is a safe and well-tolerated antimycotic drug. The transient clinical and antimycotic effect may have been due in part to the possibility that therapeutic concentrations of the drug were not reached beneath the fitting denture surface and within the denture plaque.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen C. M., Beck F. M. Strain-related differences in pathogenicity of Candida albicans for oral mucosa. J Infect Dis. 1983 Jun;147(6):1036–1040. doi: 10.1093/infdis/147.6.1036. [DOI] [PubMed] [Google Scholar]
- Beggs W. H., LaSota I. R., Hughes C. E. Is it morphologic type or physiologic state that governs susceptibility of Candida albicans to clotrimazole kill? Antimicrob Agents Chemother. 1987 Nov;31(11):1864–1865. doi: 10.1128/aac.31.11.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergendal T., Holmberg K., Nord C. E. Yeast colonization in the oral cavity and feces in patients with denture stomatitis. Acta Odontol Scand. 1979;37(1):37–45. doi: 10.3109/00016357909004683. [DOI] [PubMed] [Google Scholar]
- Bosse H. S., Blaschke-Hellmessen R. Mykologische Diagnostik und antimykotische Therapie bei Stomatitis prothetica. Zahn Mund Kieferheilkd Zentralbl. 1986;74(2):123–130. [PubMed] [Google Scholar]
- Budtz-Jorgensen E., Bertram U. Denture stomatitis. I. The etiology in relation to trauma and infection. Acta Odontol Scand. 1970 Mar;28(1):71–92. doi: 10.3109/00016357009033133. [DOI] [PubMed] [Google Scholar]
- Budtz-Jörgensen E., Löe H. Chlorhexidine as a denture disinfectant in the treatment of denture stomatitis. Scand J Dent Res. 1972;80(6):457–464. doi: 10.1111/j.1600-0722.1972.tb00314.x. [DOI] [PubMed] [Google Scholar]
- Budtz-Jörgensen E., Stenderup A., Grabowski M. An epidemiologic study of yeasts in elderly denture wearers. Community Dent Oral Epidemiol. 1975 May;3(3):115–119. doi: 10.1111/j.1600-0528.1975.tb00291.x. [DOI] [PubMed] [Google Scholar]
- Budtz-Jörgensen E., Theilade E. Regional variations in viable bacterial and yeast counts of 1-week-old denture plaque in denture-induced stomatitis. Scand J Dent Res. 1983 Aug;91(4):288–295. doi: 10.1111/j.1600-0722.1983.tb00818.x. [DOI] [PubMed] [Google Scholar]
- Budtz-Jörgensen E., Theilade E., Theilade J. Quantitative relationship between yeast and bacteria in denture-induced stomatitis. Scand J Dent Res. 1983 Apr;91(2):134–142. doi: 10.1111/j.1600-0722.1983.tb00790.x. [DOI] [PubMed] [Google Scholar]
- Budtz-Jørgensen E., Kelstrup J., Poulsen S. Reduction of formation of denture plaque by a protease (Alcalase). Acta Odontol Scand. 1983;41(2):93–98. doi: 10.3109/00016358309162308. [DOI] [PubMed] [Google Scholar]
- Gusberti F. A., Gada T. G., Lang N. P., Geering A. H. Cultivable microflora of plaque from full denture bases and adjacent palatal mucosa. J Biol Buccale. 1985 Sep;13(3):227–236. [PubMed] [Google Scholar]
- Kobayashi G. S., Travis S., Medoff G. Comparison of the in vitro and in vivo activity of the bis-triazole derivative UK 49,858 with that of amphotericin B against Histoplasma capsulatum. Antimicrob Agents Chemother. 1986 Apr;29(4):660–662. doi: 10.1128/aac.29.4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans A. S., Smitt P. A., Kalk W., de Graaff J. Efficacy of 2.5% Pimafucin suspension in the treatment of denture stomatitis. J Prosthet Dent. 1984 Apr;51(4):461–466. doi: 10.1016/0022-3913(84)90294-4. [DOI] [PubMed] [Google Scholar]
- Krogh P., Holmstrup P., Thorn J. J., Vedtofte P., Pindborg J. J. Yeast species and biotypes associated with oral leukoplakia and lichen planus. Oral Surg Oral Med Oral Pathol. 1987 Jan;63(1):48–54. doi: 10.1016/0030-4220(87)90339-2. [DOI] [PubMed] [Google Scholar]
- Odds F. C., Abbott A. B. A simple system for the presumptive identification of Candida albicans and differentiation of strains within the species. Sabouraudia. 1980 Dec;18(4):301–317. [PubMed] [Google Scholar]
- POLLACK B., BUCK I. F., KALNINS L. AN ORAL SYNDROME COMPLICATING PSYCHOPHARMACOTHERAPY: STUDY II. Am J Psychiatry. 1964 Oct;121:384–386. doi: 10.1176/ajp.121.4.384. [DOI] [PubMed] [Google Scholar]
- Perfect J. R., Savani D. V., Durack D. T. Comparison of itraconazole and fluconazole in treatment of cryptococcal meningitis and candida pyelonephritis in rabbits. Antimicrob Agents Chemother. 1986 Apr;29(4):579–583. doi: 10.1128/aac.29.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. E., Galgiani J. N. Activity of fluconazole (UK 49,858) and ketoconazole against Candida albicans in vitro and in vivo. Antimicrob Agents Chemother. 1986 Sep;30(3):418–422. doi: 10.1128/aac.30.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilade E., Budtz-Jørgensen E., Theilade J. Predominant cultivable microflora of plaque on removable dentures in patients with healthy oral mucosa. Arch Oral Biol. 1983;28(8):675–680. doi: 10.1016/0003-9969(83)90101-2. [DOI] [PubMed] [Google Scholar]
- Theilade J., Budtz-Jørgensen E. Electron microscopic study of denture plaque. J Biol Buccale. 1980 Dec;8(4):287–297. [PubMed] [Google Scholar]
- Troke P. F., Andrews R. J., Brammer K. W., Marriott M. S., Richardson K. Efficacy of UK-49,858 (fluconazole) against Candida albicans experimental infections in mice. Antimicrob Agents Chemother. 1985 Dec;28(6):815–818. doi: 10.1128/aac.28.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. M., Stafford G. D., Huggett R., Newcombe R. G. The treatment of denture-induced stomatitis. Evaluation of two agents. Br Dent J. 1981 Dec 15;151(12):416–419. doi: 10.1038/sj.bdj.4804725. [DOI] [PubMed] [Google Scholar]
- Wingard J. R., Dick J. D., Merz W. G., Sandford G. R., Saral R., Burns W. H. Differences in virulence of clinical isolates of Candida tropicalis and Candida albicans in mice. Infect Immun. 1982 Aug;37(2):833–836. doi: 10.1128/iai.37.2.833-836.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


