Abstract
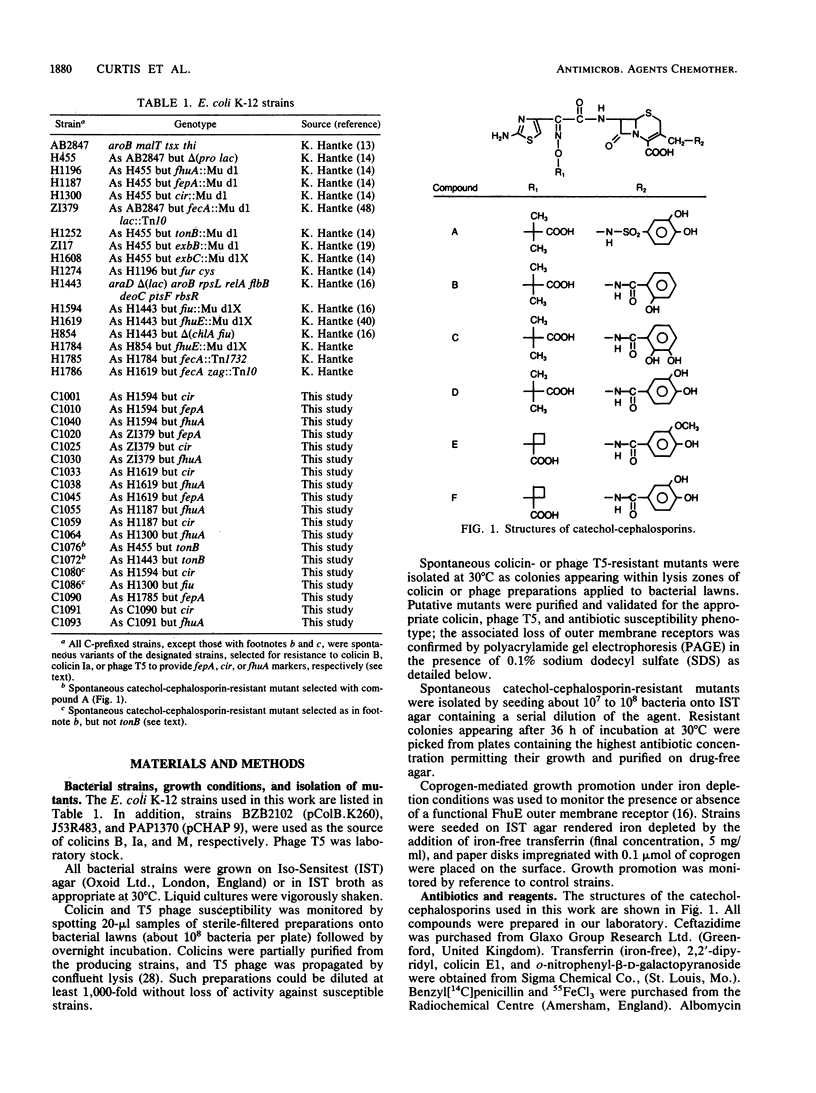
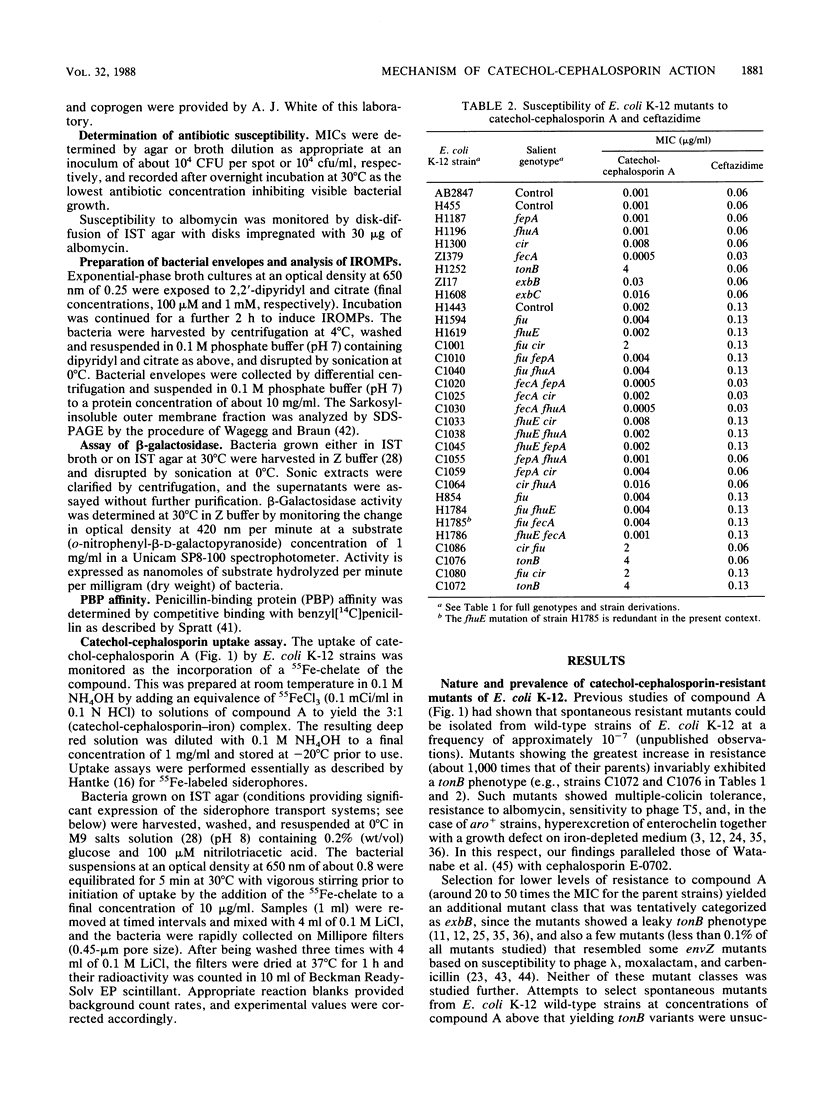
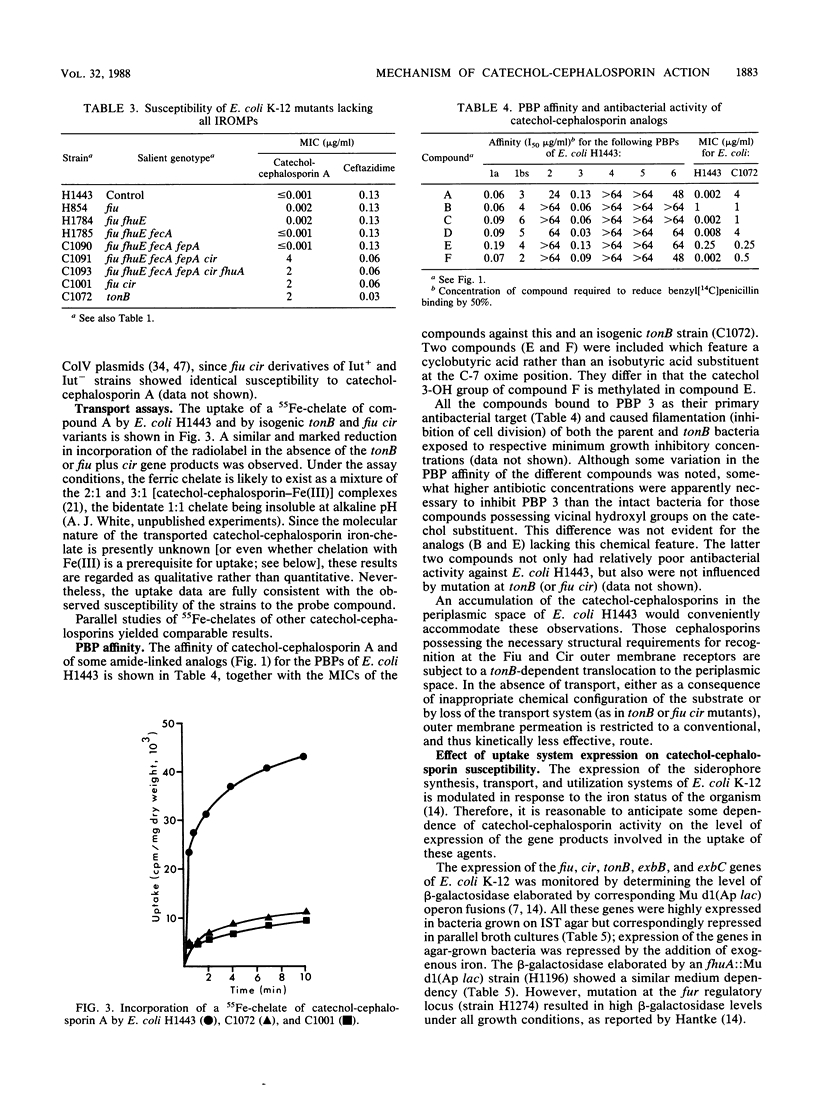
Selected aminothiazolyl-oxime cephalosporin congeners substituted at C-3' with a catechol moiety were used to probe the basis of the enhanced antibacterial activity against Escherichia coli K-12 often associated with chemical modifications of this type. Evidence is presented for a tonB-dependent illicit transport of the compounds across the outer membrane of E. coli K-12, the process involving jointly and specifically the Fiu and Cir iron-regulated outer membrane proteins. Thus, both tonB and fiu cir mutants showed a comparably reduced susceptibility to the probe compounds, whereas mutants singularly lacking any one of the six iron-regulated outer membrane proteins (Fiu, FepA, FecA, FhuA, FhuE, and Cir) or lacking any combination of any two of these proteins (except Fiu plus Cir) did not show this resistance. Mutants devoid of all six iron-regulated outer membrane proteins were no more resistant to the probe compounds than fiu cir or tonB strains. In addition to the latter genes, the products of the exbB and possibly the exbC loci were necessary for maximal antibacterial potency. A dependence of antibacterial activity on the level of expression of the uptake system components was noted. Comparison of penicillin-binding protein target affinity with antibacterial activity suggested a possible periplasmic accumulation of active compounds by E. coli K-12. Free vicinal hydroxyl groups of the catechol residue were a primary chemical requirement for recognition by the uptake pathway and thus for high antibacterial activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagg A., Neilands J. B. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry. 1987 Aug 25;26(17):5471–5477. doi: 10.1021/bi00391a039. [DOI] [PubMed] [Google Scholar]
- Basker M. J., Branch C. L., Finch S. C., Guest A. W., Milner P. H., Pearson M. J., Ponsford R. J., Smale T. C. Studies on semi-synthetic 7 alpha-formamidocephalosporins. I. Structure-activity relationships in some semi-synthetic 7 alpha-formamidocephalosporins. J Antibiot (Tokyo) 1986 Dec;39(12):1788–1791. doi: 10.7164/antibiotics.39.1788. [DOI] [PubMed] [Google Scholar]
- Braun V., Burkhardt R. Regulation of the ColV plasmid-determined iron (III)-aerobactin transport system in Escherichia coli. J Bacteriol. 1982 Oct;152(1):223–231. doi: 10.1128/jb.152.1.223-231.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton G., Best D. J., Dixon R. A., Kenyon R. F., Lashford A. G. Studies on 6 alpha-substituted penicillins. II. Synthesis and structure-activity relationships of 6 beta-(2-aryl-2-sulfoacetamido)-6 alpha-methoxy penicillanic acids. J Antibiot (Tokyo) 1986 Oct;39(10):1419–1429. doi: 10.7164/antibiotics.39.1419. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coukell M. B., Yanofsky C. Influence of chromosome structure on the frequency of tonB trp deletions in Escherichia coli. J Bacteriol. 1971 Mar;105(3):864–872. doi: 10.1128/jb.105.3.864-872.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker D. J., Matzanke B. F., Raymond K. N. Recognition and transport of ferric enterobactin in Escherichia coli. J Bacteriol. 1986 Aug;167(2):666–673. doi: 10.1128/jb.167.2.666-673.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick-Helmerich K., Hantke K., Braun V. Cloning and expression of the exbB gene of Escherichia coli K-12. Mol Gen Genet. 1987 Feb;206(2):246–251. doi: 10.1007/BF00333580. [DOI] [PubMed] [Google Scholar]
- Guterman S. K., Dann L. Excretion of enterochelin by exbA and exbB mutants of Escherichia coli. J Bacteriol. 1973 Jun;114(3):1225–1230. doi: 10.1128/jb.114.3.1225-1230.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Functional interaction of the tonA/tonB receptor system in Escherichia coli. J Bacteriol. 1978 Jul;135(1):190–197. doi: 10.1128/jb.135.1.190-197.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K. Cloning of the repressor protein gene of iron-regulated systems in Escherichia coli K12. Mol Gen Genet. 1984;197(2):337–341. doi: 10.1007/BF00330982. [DOI] [PubMed] [Google Scholar]
- Hantke K. Identification of an iron uptake system specific for coprogen and rhodotorulic acid in Escherichia coli K12. Mol Gen Genet. 1983;191(2):301–306. doi: 10.1007/BF00334830. [DOI] [PubMed] [Google Scholar]
- Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182(2):288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- Heidinger S., Braun V., Pecoraro V. L., Raymond K. N. Iron supply to Escherichia coli by synthetic analogs of enterochelin. J Bacteriol. 1983 Jan;153(1):109–115. doi: 10.1128/jb.153.1.109-115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein S., Hantke K., Braun V. Citrate-dependent iron transport system in Escherichia coli K-12. Eur J Biochem. 1981 Jul;117(2):431–437. doi: 10.1111/j.1432-1033.1981.tb06357.x. [DOI] [PubMed] [Google Scholar]
- Jaffé A., Chabbert Y. A., Derlot E. Selection and characterization of beta-lactam-resistant Escherichia coli K-12 mutants. Antimicrob Agents Chemother. 1983 Apr;23(4):622–625. doi: 10.1128/aac.23.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konisky J. Colicins and other bacteriocins with established modes of action. Annu Rev Microbiol. 1982;36:125–144. doi: 10.1146/annurev.mi.36.100182.001013. [DOI] [PubMed] [Google Scholar]
- Lundrigan M., Earhart C. F. Reduction in three iron-regulated outer membrane proteins and protein a by the Escherichia coli K-12 perA mutation. J Bacteriol. 1981 May;146(2):804–807. doi: 10.1128/jb.146.2.804-807.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzanke B. F., Ecker D. J., Yang T. S., Huynh B. H., Müller G., Raymond K. N. Escherichia coli iron enterobactin uptake monitored by Mössbauer spectroscopy. J Bacteriol. 1986 Aug;167(2):674–680. doi: 10.1128/jb.167.2.674-680.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida K., Ono Y., Yamasaki M., Shiraki C., Hirata T., Sato K., Okachi R. Aminothiazolylglycyl derivatives of carbacephem antibiotics. II. Synthesis and antibacterial activity of novel aminothiazolyl cephem compounds with hydroxypyridone moiety. J Antibiot (Tokyo) 1987 Feb;40(2):182–189. doi: 10.7164/antibiotics.40.182. [DOI] [PubMed] [Google Scholar]
- Mochida K., Shiraki C., Yamasaki M., Hirata T., Sato K., Okachi R. Aminothiazolylglycyl derivatives of carbacephems. I. Synthesis and antibacterial activity of novel carbacephems with substituted aminothiazolyl groups. J Antibiot (Tokyo) 1987 Jan;40(1):14–21. doi: 10.7164/antibiotics.40.14. [DOI] [PubMed] [Google Scholar]
- Nakagawa S., Sanada M., Matsuda K., Hazumi N., Tanaka N. Biological activity of BO-1236, a new antipseudomonal cephalosporin. Antimicrob Agents Chemother. 1987 Jul;31(7):1100–1105. doi: 10.1128/aac.31.7.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Ohi N., Aoki B., Shinozaki T., Moro K., Kuroki T., Noto T., Nehashi T., Matsumoto M., Okazaki H., Matsunaga I. Semisynthetic beta-lactam antibiotics. IV. Synthesis and antibacterial activity of new ureidocephalosporin and ureidocephamycin derivatives containing a catechol moiety or its acetate. Chem Pharm Bull (Tokyo) 1987 May;35(5):1903–1909. [PubMed] [Google Scholar]
- Ohi N., Aoki B., Shinozaki T., Moro K., Noto T., Nehashi T., Okazaki H., Matsunaga I. Semisynthetic beta-lactam antibiotics. I. Synthesis and antibacterial activity of new ureidopenicillin derivatives having catechol moieties. J Antibiot (Tokyo) 1986 Feb;39(2):230–241. doi: 10.7164/antibiotics.39.230. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Reeves P. Characterization of group B colicin-resistant mutants of Escherichia coli K-12: colicin resistance and the role of enterochelin. J Bacteriol. 1976 Jul;127(1):218–228. doi: 10.1128/jb.127.1.218-228.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Reeves P. Iron uptake in colicin B-resistant mutants of Escherichia coli K-12. J Bacteriol. 1976 Jun;126(3):1052–1062. doi: 10.1128/jb.126.3.1052-1062.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Zimmerman W., Wehrli W. Highly efficient uptake of a rifamycin derivative via the FhuA-TonB-dependent uptake route in Escherichia coli. J Gen Microbiol. 1987 Dec;133(12):3505–3511. doi: 10.1099/00221287-133-12-3505. [DOI] [PubMed] [Google Scholar]
- Reynolds P. R., Mottur G. P., Bradbeer C. Transport of vitamin B12 in Escherichia coli. Some observations on the roles of the gene products of BtuC and TonB. J Biol Chem. 1980 May 10;255(9):4313–4319. [PubMed] [Google Scholar]
- Sanders C. C. Chromosomal cephalosporinases responsible for multiple resistance to newer beta-lactam antibiotics. Annu Rev Microbiol. 1987;41:573–593. doi: 10.1146/annurev.mi.41.100187.003041. [DOI] [PubMed] [Google Scholar]
- Sauer M., Hantke K., Braun V. Ferric-coprogen receptor FhuE of Escherichia coli: processing and sequence common to all TonB-dependent outer membrane receptor proteins. J Bacteriol. 1987 May;169(5):2044–2049. doi: 10.1128/jb.169.5.2044-2049.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Wagegg W., Braun V. Ferric citrate transport in Escherichia coli requires outer membrane receptor protein fecA. J Bacteriol. 1981 Jan;145(1):156–163. doi: 10.1128/jb.145.1.156-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C., Moreno F., Schwartz M. Pleiotropic mutations rendering Escherichia coli K-12 resistant to bacteriophage TP1. J Bacteriol. 1980 Sep;143(3):1374–1383. doi: 10.1128/jb.143.3.1374-1383.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., Sarthy A., Beckwith J. Escherichia coli pleiotropic mutant that reduces amounts of several periplasmic and outer membrane proteins. J Bacteriol. 1979 Oct;140(1):229–239. doi: 10.1128/jb.140.1.229-239.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N. A., Nagasu T., Katsu K., Kitoh K. E-0702, a new cephalosporin, is incorporated into Escherichia coli cells via the tonB-dependent iron transport system. Antimicrob Agents Chemother. 1987 Apr;31(4):497–504. doi: 10.1128/aac.31.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H. Novel iron uptake system specified by ColV plasmids: an important component in the virulence of invasive strains of Escherichia coli. Infect Immun. 1979 Dec;26(3):925–932. doi: 10.1128/iai.26.3.925-932.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann L., Hantke K., Braun V. Exogenous induction of the iron dicitrate transport system of Escherichia coli K-12. J Bacteriol. 1984 Jul;159(1):271–277. doi: 10.1128/jb.159.1.271-277.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo V., Wee S., Herrero M., Neilands J. B. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol. 1987 Jun;169(6):2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



