Abstract
Over the past decade, discussions of the evolution of the earliest human ancestors have focused on the locomotion of the australopithecines. Recent discoveries in a broad range of disciplines have raised important questions about the influence of ecological factors in early human evolution. Here we trace the cranial and dental traits of the early australopithecines through time, to show that between 4.4 million and 2.3 million years ago, the dietary capabilities of the earliest hominids changed dramatically, leaving them well suited for life in a variety of habitats and able to cope with significant changes in resource availability associated with long-term and short-term climatic fluctuations.
Since the discovery of Australopithecus afarensis, many researchers have emphasized the importance of bipedality in scenarios of human origins (1, 2). Surprisingly, less attention has been focused on the role played by diet in the ecology and evolution of the early hominids (as usually received). Recent work in a broad range of disciplines, such as paleoenvironmental studies (3, 4), behavioral ecology (5), primatology (6), and isotope analyses (7), has rekindled interests in early hominid diets. Moreover, important new fossils from the early Pliocene raise major questions about the role of dietary changes in the origins and early evolution of the Hominidae (8–10). In short, we need to focus not just on how the earliest hominids moved between food patches, but also on what they ate when they got there.
This paper presents a review of the fossil evidence for the diets of the Pliocene hominids Ardipithecus ramidus, Australopithecus anamensis, Australopithecus afarensis, and Australopithecus africanus. These hominids offer evidence for the first half of human evolution, from our split with prehistoric apes to the earliest members of our own genus, Homo. The taxa considered are viewed as a roughly linear sequence from Ardipithecus to A. africanus, spanning the time from 4.4 million to 2.5 million years ago. As such, they give us a unique opportunity to examine changes in dietary adaptations of our ancestors over nearly 2 million years. We also trace what has been inferred concerning the diets of the Miocene hominoids to put changes in Pliocene hominid diets into a broader temporal perspective. From such a perspective, it becomes clear that the dietary capabilities of the early hominids changed dramatically in the time period between 4.4 million and 2.3 million years ago. Most of the evidence has come from five sources: analyses of tooth size, tooth shape, enamel structure, dental microwear, and jaw biomechanics. Taken together, they suggest a dietary shift in the early australopithecines, to increased dietary flexibility in the face of climatic variability. Moreover, changes in diet-related adaptations from A. anamensis to A. afarensis to A. africanus suggest that hard, abrasive foods became increasingly important through the Pliocene, perhaps as critical items in the diet.
Tooth Size
In 1970, Jolly (11) noted that australopithecines had relatively small incisors compared with molars and speculated that this might be associated with terrestrial seed eating, as seen in Theropithecus today. Although this idea has been the subject of some controversy (12), Jolly's efforts have stimulated considerable research on the origins of hominid adaptations and on relative incisor size in a wide variety of living and fossil primates. Hylander (13), for example, examined the relationship of incisor row length (relative to body size) in a range of living anthropoids and found that those species with larger incisors tend to consume larger, tougher fruits, whereas those with smaller front teeth tend to feed on smaller foods, or those that require less extensive incisal preparation, such as leaves or berries. Since the work of Jolly and Hylander, numerous workers have looked to incisor size in early hominids and other fossil primates for clues concerning diet.
What can incisor size tell us of the diets of Miocene apes? Unfortunately, not as much as one would like. Ideally, to consider relative incisor sizes among taxa, we need estimates of species body weights based on attributes independent of the dentition. Such estimates are unavailable for most taxa. Furthermore, Miocene apes as a whole evidently had small incisors compared with extant hominoids, in much the same way that platyrrhines as a whole have relatively smaller incisors than do catarrhines, regardless of diet (14). Such phylogenetic effects make it difficult to find an extant comparative baseline series with which to compare these basal taxa of uncertain phyletic affinities.
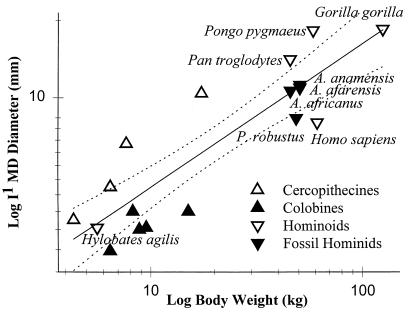
On the other hand, incisor size might give us some clues to diet and tooth use for the early australopithecines, and we have good, consistent weight estimates from independent studies (15, 16) for many of these taxa. If we look at a regression of maxillary central incisor breadth on body size for species representing a variety of catarrhine genera, we see a separation of cercopithecines (with relatively larger incisors) above the line and colobines below (Fig. 1). Furthermore, more frugivorous chimpanzees and orangutans fall above the line, whereas gibbons and gorillas fall close to the line, with relatively smaller incisors. Indeed, values for the living frugivorous great apes fall above the 95% confidence limits of expected incisor size for modern catarrhines. The human values fall below the 95% confidence limits, indicating that we have very small incisors relative to body size.
Figure 1.
Relative maxillary first incisor sizes in catarrhines. MD, mesiodistal. Dashed lines indicate 95% confidence limits of the least-squares regression plot (data from refs. 15 and 17–20).
Relative incisor sizes for the three “gracile” australopithecines are remarkably similar, and they fall very close to the regression line, much like the gorilla. These results are similar to those reported by Kay (21) and Ungar and Grine (17) and suggest that these hominids used their incisors in ingestion to a similar degree, although they all probably used these teeth less than either the chimpanzee or orangutan. These data can also give us some idea of whether a taxon often eats foods that require incisal preparation. For instance, lar gibbons have much smaller incisors than orangutans, and they depend on smaller fruits requiring little incisal preparation (17, 22, 23). From this perspective, the australopithecines probably put less emphasis on foods that require substantial incisor use, such as those with thick husks and those with flesh adherent to large, hard seeds. Body weight estimates and incisor size data for Ardipithecus ramidus and Australopithecus garhi should provide even more insights.
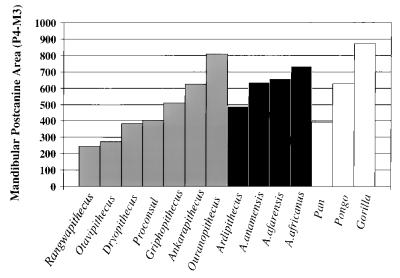
One of the hallmarks of the australopithecines has always been their large, relatively flat molars (24–29). There are certainly differences in the amount of occlusal relief between gracile and robust australopithecines (30) (see below). However, by comparison with other primates, the australopithecines' molars are still flat and huge. Even in the earliest hominids, this can be seen in a simple plot of mandibular postcanine tooth area (MD × BL, the product of maximal mesiodistal and buccolingual diameters), where most taxa have teeth larger than those of the modern orangutan (Fig. 2).
Figure 2.
Summed mandibular postcanine tooth areas (P4–M3) in Miocene apes, early hominids, and extant apes (data from refs. 8, 18–20, and 31–37).
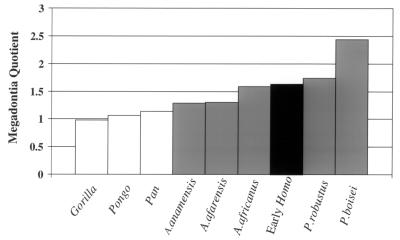
The only exception is Ardipithecus, which is more chimp-sized in the P4–M1 region, but intermediate between chimpanzees and orangutans in the M2–M3 region. Again, interpretations of such differences are hampered by the lack of body size estimates for Ardipithecus, but if a body size estimate of 51 kg is used for A. anamensis (the average of the two different estimates based on the tibia) (18), McHenry's “megadontia quotient” for this taxon is essentially identical to that for A. afarensis (Fig. 3). In other words, its molars are large for a hominoid, but smaller than those of A. africanus or the “robust” australopithecines.
Figure 3.
Megadontia quotients for early hominids and extant primates (data from refs. 18, 20, 27, 31, and 38).
As one might expect, the Miocene hominoids show a tremendous range of mandibular molar sizes (Fig. 2). Many have postcanine tooth areas larger than that of Ardipithecus, and some (such as Ouranopithecus) even have larger postcanine tooth areas than that of A. anamensis, but as all body size estimates for them have been computed from dental remains, a megadontia quotient cannot be computed. The main message from a simple look at postcanine tooth size is that the earliest hominids make a nice progression leading into subsequent hominids, but they do not have larger postcanine teeth than all of the middle to late Miocene hominoids.
This might just mean that there are a variety of body sizes sampled in these taxa. However, as shown by the work of Lucas and colleagues (39), variations in tooth size are a means of adapting to changes in the external characteristics of foods, such as their size, shape, and abrasiveness. Clearly, some of these food characteristics were changing during the evolution of the earliest hominids, as postcanine teeth became relatively larger and larger. However, evidence from the middle to late Miocene shows that tooth size, by itself, cannot pinpoint the initial change to a hominid diet, at least not with the samples at hand.
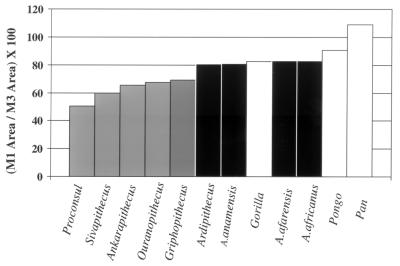
One other way of looking at postcanine tooth size is to look at the ratio of the areas of M1 and M3 (Fig. 4). Lucas et al. (39) showed that this ratio was inversely related to the percentage of leaves, flowers, and shoots in the diet; that is, anthropoids with a high ratio of M1 to M3 area consumed more fruit than did those with a low M1 to M3 ratio. When this is computed for the earliest hominids, plus a sample of Miocene apes, a clear separation is evident, with the early hominids, including Ardipithecus, showing higher ratios than the Miocene apes. So, does this indicate more fruit in the diet of the earliest hominids? To begin to answer this question, we must look at analyses of tooth shape.
Figure 4.
Ratios of M1 to M3 areas, defined as the products of maximal mesiodistal and buccolingual diameters (data from refs. 8, 18–20, and 31–37).
Tooth Shape
Variations in tooth shape are a means of adapting to changes in the internal characteristics of foods, such as their strength, toughness, and deformability (39–43). Clearly, foods are complicated structures; thus it is impossible to describe all of the internal characteristics that might have confronted the earliest hominids' teeth. However, another approach is to describe the capabilities of those teeth.
For example, tough foods, those that are difficult to fracture, are generally sheared between the leading edges of sharp crests. In contrast, hard brittle foods, those that are easy to fracture but difficult to penetrate, are crushed between planar surfaces. As such, reciprocally concave, highly crested teeth have the capability of efficiently processing tough items such as insect exoskeletons and leaves, whereas rounder and flatter cusped teeth are best suited for a more frugivorous diet. Kay (21) has devised a “shearing quotient” as a measure of the relative shear potential of molar teeth. Basically, more folivorous species have the highest shearing quotients, followed by those that prefer brittle, soft fruits; finally, hard-object feeders have the lowest shearing quotients (21, 44).
Shearing crest studies have been conducted on early Miocene African apes and middle to late Miocene European apes. These studies suggest a considerable range of diets in these forms. For example, Rangwapithecus and Oreopithecus have relatively long shearing crests, suggesting folivory; Ouranopithecus has extremely short “crests,” suggesting a hard-object specialization; whereas most other Miocene taxa studied, such as Proconsul and Dryopithecus, have the intermediate length crests of a frugivore (14, 45).
As for the early hominids, A. africanus had more occlusal relief than did Paranthropus robustus, suggesting a dietary difference between these species (30). Additional preliminary shearing quotient studies support this idea while reaffirming that the australopithecines, as a group, had relatively flat, blunt molar teeth and lacked the long shearing crests seen in some extant hominoids (28). By itself, this indicates that the earliest hominids would have had difficulty breaking down tough, pliant foods, such as soft seed coats and the veins and stems of leaves—although they probably were capable of processing buds, flowers, and shoots.
Interestingly, as suggested by Lucas and Peters (46), another tough pliant food they would have had difficulty processing is meat. In other words, the early hominids were not dentally preadapted to eat meat—they simply did not have the sharp, reciprocally concave shearing blades necessary to retain and cut such foods. In contrast, given their flat, blunt teeth, they were admirably equipped to process hard brittle objects. What about soft fruits? It really depends on the toughness of those fruits. If they were tough, then they would also need to be precisely retained and sliced between the teeth. Again, early hominids would be very inefficient at it. If they were not tough, then the hominids could certainly process soft fruits.
In sum, Miocene apes show a range of adaptations, including folivory, soft-fruit eating, and hard-object feeding. This range exceeds that of living hominoids and especially the early hominids. Although studies of shearing crest length have been conducted on only some of the early hominids, all evidence indicates that the australopithecines had relatively flat molar teeth compared with many living and fossil apes. These teeth were well suited for breaking down hard, brittle foods, including some fruits and nuts, and soft, weak foods, such as flowers and buds; but again, they were not well suited for breaking down tough pliant foods such as stems, soft seed pods, and meat.
Enamel Structure
Another area of interest regarding dental functional anatomy is the study of enamel thickness. There are certainly methodological differences between studies (47–52), but the consensus still seems to be that the australopithecines had relatively thick enamel compared with living primates, and that many of the Miocene apes also had thick enamel (24, 28, 48–49, 51, 53–54). Interestingly, this perspective may be changing as we get glimpses of more and more new taxa. For instance, Conroy et al. (55) have noted that Otavipithecus may have had thin enamel, and White et al. (8) have made the same observation for Ardipithecus. Granted, in neither case do we have a detailed series of measurements over the tooth crown, but still, the figures that have been quoted (less than 1 mm for Otavipithecus and 1.1–1.2 mm for Ardipithecus) are far less than those quoted for the australopithecines.
So what might be the functional significance of enamel thickness? The most frequently cited correlations are between the consumption of hard food items, or abrasive food items, and thick molar enamel (58–59). There are many potential complicating factors (51, 56, 59–60); thus it is perhaps not surprising that the correlation between enamel thickness and diet is not a perfect one (57). Moreover, thick enamel by itself does not necessarily provide protection against hard objects, which commonly cause fracture of enamel (61). The best protection against this is prism or crystallite decussation or interweaving. Maas (62, 63), Rensberger (64, 65), and others (42, 59) have shown that prism and crystallite orientations can give clues to intricate details of dental function, and that decussation can be an effective crack-stopping mechanism in many animals. Only anecdotal references to this phenomenon in Miocene apes and early hominids have been made thus far, largely because more detailed work generally requires the sectioning and etching of teeth. Still, after some discussion and debate (48–49, 53), a consensus now seems to be that they did have a significant degree of prism decussation. Thus, the thick enamel of the early hominids may have been a means of resisting breakage during the consumption of hard objects and an adaptation that prolonged the life of the tooth, given an abrasive diet.
Dental Microwear
Numerous workers have recognized that microscopic wear on the incisors and molars of primates reflects tooth use and diet. For example, those primates that often use their front teeth in ingestion have high densities of microwear striations on their incisors. Furthermore, folivores have a high incidences of long narrow scratches on their molars, whereas frugivores have more pits on those surfaces. Among frugivores, hard-object feeders have even higher pit incidences than soft-fruit eaters. These and other relationships between microwear and feeding behaviors in living primates have been used to infer diet in fossil forms. Miocene apes have a remarkable range of microwear patterning, greatly exceeding that of living hominoids. For example, relatively high scratch densities suggest that Micropithecus, Rangwapithecus, and especially Oreopithecus (66) included more leaves in their diets. In contrast, high pit percentages suggest that Griphopithecus and Ouranopithecus (66) were hard-object specialists. Finally, intermediate microwear patterns suggest that most other species studied, such as Gigantopithecus, Dendropithecus, Proconsul, Dryopithecus, and, perhaps, Sivapithecus (66–68), had diets dominated by soft fruits. These data give us a glimpse of the extraordinary variation from which the last common ancestor of apes and hominids evidently arose.
Unfortunately, little is known about the microwear of early australopithecines. No microwear research has yet been published for either Ardipithecus ramidus or A. anamensis, although there has been some done on A. afarensis and A. africanus. The work done on A. afarensis has been largely qualitative and focused on the anterior teeth, and it suggests that these hominids were beginning to exploit savanna resources (69). Furthermore, Ryan and Johanson (70) argued that A. afarensis had a mosaic of gorilla-like fine wear striae and baboon-like pits and microflakes, indicating the use of incisors to strip gritty plant parts such as seeds, roots, and rhizomes. These authors also suggested that there was a functional shift in the P3 complex from ape-like slicing and cutting to hominid puncture-crushing.
Work done on A. africanus has been more quantitative but has focused on comparing this taxon to Paranthropus robustus rather than to extant hominoids. Grine (71) found that A. africanus molars have lower incidences of pitting than seen for Paranthropus. A. africanus scratches are also longer and narrower and show more homogeneity in orientation. Grine argued that compared with the “robust” forms, A. africanus ate more soft fruits and leaves. Comparisons with work from Teaford (72) places A. africanus between Cebus olivaceus on one hand and Pan troglodytes on the other. Work on A. africanus incisors has shown that this taxon has higher microwear feature densities on all surfaces examined than does Paranthropus (17). This suggests that A. africanus processed a greater variety of foods with its front teeth, including larger, more abrasive ones, than were encountered by Paranthropus. Comparisons with an extant baseline series examined by Ungar (73) puts Australopithecus between Pongo pygmaeus and the seed predator/folivore Presbytis thomasi in degree of anterior tooth use in ingestion.
In sum, then, the microwear suggests that, by the end of the Miocene, hominoids had a wide range of diets. In contrast, A. afarensis probably focused on soft fruit but also began to incorporate into its diet abrasive, terrestrial resources that required incisal stripping. A. africanus may still have focused on soft fruit, particularly that which required a moderate amount of incisal preparation. Clearly, considerably more work is needed on these and other early hominids to put together a reasonable picture of diet based on microwear evidence.
Mandibular Biomechanics
Finally, there are other lines of evidence that we can examine to look for evidence of diet. Mandibular fragments are among the most common bony remains found at hominid fossil sites, and the architecture of this bone has been adapted to withstand stresses and strains associated with oral food processing. Thus its morphology probably reflects some aspects of diet. Analyses of australopithecine mandibular biomechanics have focused on corpus size and shape.
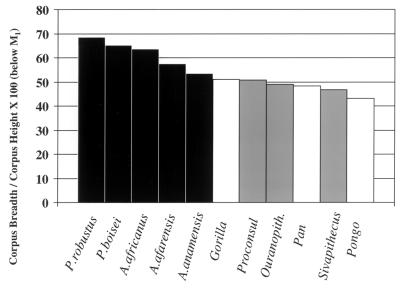
Comparisons with extant hominoids have shown that A. afarensis and A. africanus have relatively thick mandibular corpora (74, 75). The same pattern was also found for Paranthropus boisei and P. robustus. Fig. 5 shows mandibular robusticity index values for extant great apes, some Miocene apes, and early australopithecines. The early hominids show relatively thicker mandibular corpora than extant great apes and Miocene catarrhines, suggesting a morphological shift in the former.
Figure 5.
Mandibular corpus shape (data from refs. 75, 76, and 85 and M. Leakey, personal communication).
Both functional and nonfunctional interpretations have been offered to explain this phenomenon. For example, it may simply be that a thick mandibular corpus is an effect of large cheek teeth or a reduced canine. This is not a likely explanation, however, as australopithecines still have relatively broad mandibles when considered relative to molar size, and there appears to be no relationship between mandibular robusticity and relative canine size among the australopithecines (75).
Despite some inherent difficulties, it seems more likely that the unique shape of the australopithecine mandibular corpus relates to the functional demands of mastication. Thickened mandibles can act to resist extreme stresses associated with transverse bending (that is, “wishboning”) and torsion. Because wishboning stresses decline toward the back of the corpus, torsion is likely a more important explanation. Corpus torsion can result from bite force and muscle activity during mastication. Therefore, it may be that australopithecine mandibular morphology reflects elevated stresses associated with unusual mechanical demands. Daegling and Grine (75) suggest that australopithecines may have eaten fibrous, coarse foods that required repetitive loading. While this fails to explain why colobines do not have thick corpora, it does suggest a fundamental difference between australopithecines and living great apes that may reflect a shift in diet in the early hominids.
Studies of corpus shape in A. anamensis and Ardipithecus ramidus will likely provide further clues regarding differences in mandibular architecture between great apes and later australopithecines. Corpus robusticity indices for A. anamensis below M1 average 53.5 (M. Leakey, personal communication). These values fall at the upper range for extant hominoids (Pan = 39.2–57.8; Gorilla = 43.5–59.7; Pongo = 35.7–52.0) and at the lower end of the range for later fossil hominids (A. afarensis = 48.4–68.9, A. africanus = 54.8–79.0) (Fig. 5) (data from Daegling and Grine and Lockwood et al.) (75, 85).
In sum, the architecture of the mandibular corpus suggests that the “gracile” australopithecines differed from living apes in their abilities to dissipate masticatory stresses. Taken with other lines of evidence, this certainly suggests a difference in diet between living apes and A. anamensis, and between A. anamensis and later hominids, with A. anamensis intermediate between the African ape and later australopithecine conditions.
Discussion
The australopithecines exhibited a complex of morphological features related to diet that are unique compared with living hominoids or Miocene apes. These early hominids all had small- to moderate-sized incisors; large, flat molars with little shear potential; a ratio of first to third molar area that was low compared with those of extant apes, but generally higher than those of Miocene apes; thick tooth enamel; and thick mandibular corpora. This suite of traits is distinctive of australopithecines and suggests a dietary shift at or near the stem of hominid evolution. Their thick-enameled, flattened molars would have had great difficulty propagating cracks through tough foods, suggesting that the australopithecines were not well suited for eating tough fruits, leaves, or meat. The dental microwear data agree with this conclusion, as the australopithecine patterns documented to date are most similar to those of modern-day seed predators and soft fruit eaters. Furthermore, given their comparatively small incisors, these hominids probably did not specialize in large, husked fruits or those requiring extensive incisal preparation. Instead, the australopithecines would have easily been able to break down hard, brittle foods. Their large flat molars would have served well for crushing, and their thick enamel would have withstood abrasion and fracture. Their mandibular corpora would probably have conferred an advantage for resisting failure, given high occlusal loads. In essence, for much of their history, the australopithecines had an adaptive package that allowed them ready access to hard objects, plus soft foods that were not particularly tough. The early hominids could also have eaten both abrasive and nonabrasive foods. This ability to eat both hard and soft foods, plus abrasive and nonabrasive foods, would have left the early hominids particularly well suited for life in a variety of habitats, ranging from gallery forest to open savanna.
Does this mean we can talk of a characteristic “australopithecine” dietary pattern? Perhaps to some extent, but although the australopithecines shared many features in common, they also differed from one another, suggesting a change in diet through time. Such morphological changes occurred as a mosaic, much as that seen for locomotor anatomy.
Much of the evidence for Ardipithecus ramidus is not yet available, but despite its thin molar enamel and absolutely smaller teeth than those of later hominids, it shows molar size proportions that may hint at dietary changes to come. A. anamensis shows the first indications of thicker molar enamel in a hominid, and its molar teeth were equivalent in size to those of A. afarensis. Still, its mandibular corpus is intermediate in robusticity between those of living great apes and later australopithecines. This combination of features suggests that A. anamensis might have been the first hominid to be able to effectively withstand the functional demands of hard and perhaps abrasive objects in its diet, whether or not such items were frequently eaten or were only an important occasional food source. A. afarensis was similar to A. anamensis in relative tooth sizes and probably enamel thickness, yet it did show a large increase in mandibular robusticity. This increase may be due to changes in peak force magnitude or degree of repetitive loading in mastication. Either way, hard and perhaps abrasive foods may have become even more important components of the diet of A. afarensis. A. africanus shows yet another increase in postcanine tooth size, which by itself would suggest an increase in the sizes and abrasiveness of foods. However, its molar microwear does not show the degree of pitting one might expect from a classic hard-object feeder. Thus, even A. africanus has evidently not begun to specialize in hard objects, but rather has emphasized dietary breadth. In contrast, subsequent “robust” australopithecines do show hard-object microwear and craniodental specializations, suggesting a substantial departure in feeding adaptive strategies early in the Pleistocene.
In sum, diet was probably an important factor in the origin and early evolution of our family. The earliest australopithecines show a unique suite of diet-related features unlike those of Miocene apes or living hominoids. Such features suggest that the earliest hominids may have begun to experiment with harder, more brittle foods at the expense of softer, tougher ones early on. This does not mean that all of the australopithecines were specialized hard-object feeders. It merely means that, through time, they acquired the ability to feed on hard objects. Many modern primates need to consume critical “fall-back foods” at certain times of the year (6), and it may well be that the earliest australopithecines resorted to the consumption of hard objects only in such situations, whereas the robust australopithecines relied on them far more regularly.
Another important aspect of early hominid trophic adaptations is evident from data presented here—the dietary shift from apes to early hominids did not involve an increase in the consumption of tough foods, and so the australopithecines were not preadapted for eating meat. This conclusion runs counter to (i) recent isotope work suggesting that the australopithecines did in fact consume significant amounts of meat (7) and (ii) nutritional work suggesting that meat may have provided critical nutrients for both young and old hominids (77–79). There would seem to be three different ways to reconcile these perspectives. First, the present study has reviewed only craniodental features related to diet. If the australopithecines used other means for ingesting and processing meat (e.g., tools), they might have been able to process meat more efficiently than the craniodental evidence suggests (80, 81). Second, the heavy C3 signature found in A. africanus (7) may reflect the consumption of underground storage organs of C3 plants rather than meat (82). Third, the functional analyses of the teeth assume that all meat has the same degree of toughness. This may not be the case. Studies of the physical properties of food have thus far focused on plant remains, with only brief mention of the toughness of materials like skin (40, 46). Variations in toughness between animal tissues might well be due to variations in the arrangement and density of collagen matrix. Furthermore, the physical effects of decomposition might render meat less tough and more readily processed by hominids. If this is so, it could be further evidence in support of scavenging as part of the early hominid way of life.
Investigators have tried to relate patterns of hominid evolution to patterns of climatic change for some time (3, 4). The focus of much of the recent work has been on the origin of the genus Homo. Can the dietary shifts in the earliest hominids also be tied to such changes? Whereas there is some evidence of large-scale climatic changes around the Mediterranean (83) and unusual faunal turnover in parts of western Asia (84), there are no large-scale changes evident in sub-Saharan Africa until after the earliest hominids have arrived on the scene (i.e., not until 1.5–2.5 million years ago). There is the slow and inexorable cooling and drying of the Miocene, but perhaps the crucial result of this was an increase in microhabitat variability. Certainly, there are limits to our paleoecological evidence from this period, but as Potts (4) has noted, “in general, the oldest hominids were associated with a diverse range of habitats.” These included lake and river margins, woodland, bushland, and savanna. Potts (4) has emphasized that locomotor versatility was a crucial adaptation of the earliest hominids in the face of such varied environmental conditions. We feel that this perspective needs to be extended to the dietary adaptations of the earliest hominids as well. In such a land of variable opportunities, the generalized craniodental toolkit of the earliest hominids may have had a distinct advantage, as it allowed our forbears the flexibility to cope with short-term and long-term climatic variations and the resultant changes in resource availability.
Acknowledgments
We are grateful to the Governments of Ethiopia, Kenya, and Tanzania and especially to the National Museums of Ethiopia, Kenya, and Tanzania for permission to study early hominid specimens in their care. This work was supported by National Science Foundation Grants SBR 9804882 and 9601766.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.260368897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.260368897
References
- 1.Lovejoy C O. In: Primate Functional Morphology and Evolution. Tuttle R L, editor. The Hague: Mouton; 1975. pp. 291–326. [Google Scholar]
- 2.Susman R L, Stern J T, Jungers W L. Folia Primatol. 1984;43:113–156. doi: 10.1159/000156176. [DOI] [PubMed] [Google Scholar]
- 3.Vrba E S. In: Paleoclimate and Evolution, with Emphasis on Human Origins. Vrba E S, Denton G H, Partridge T C, Burckle L H, editors. New Haven, CT: Yale Univ. Press; 1995. pp. 24–45. [Google Scholar]
- 4.Potts R. Yearbook Phys Anthropol. 1998;41:93–136. doi: 10.1002/(sici)1096-8644(1998)107:27+<93::aid-ajpa5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 5.O'Connell J F, Hawkes K, Blurton Jones N G. J Hum Evol. 1999;36:461–485. doi: 10.1006/jhev.1998.0285. [DOI] [PubMed] [Google Scholar]
- 6.Conklin-Brittain N L, Wrangham R W, Hunt K D. Int J Primatol. 1998;19:949–970. [Google Scholar]
- 7.Sponheimer M, Lee-Thorp J A. Science. 1999;283:368–370. doi: 10.1126/science.283.5400.368. [DOI] [PubMed] [Google Scholar]
- 8.White T D, Suwa G, Asfaw B. Nature (London) 1994;371:306–312. doi: 10.1038/371306a0. [DOI] [PubMed] [Google Scholar]
- 9.Asfaw B, White T, Lovejoy O, Latimer B, Simpson S, Suwa G. Science. 1999;284:629–634. doi: 10.1126/science.284.5414.629. [DOI] [PubMed] [Google Scholar]
- 10.Ward C, Leakey M, Walker A. Evol Anthropol. 1999;7:197–205. [Google Scholar]
- 11.Jolly C J. Man. 1970;5:1–26. [Google Scholar]
- 12.Dunbar R I M. J Hum Evol. 1976;5:161–167. [Google Scholar]
- 13.Hylander W L. Science. 1975;189:1095–1098. doi: 10.1126/science.808855. [DOI] [PubMed] [Google Scholar]
- 14.Kay R F, Ungar P S. In: Function, Phylogeny and Fossils: Miocene Hominoids and Great Ape and Human Origins. Begun D R, Ward C, Rose M, editors. New York: Plenum; 1997. pp. 131–151. [Google Scholar]
- 15.Jungers W L. In: Evolutionary History of the “Robust” Australopithecines. Grine F E, editor. New York: de Gruyter; 1988. pp. 115–125. [Google Scholar]
- 16.McHenry H M. Evol Anthropol. 1992;1:15–20. [Google Scholar]
- 17.Ungar P S, Grine F E. J Hum Evol. 1991;20:313–340. [Google Scholar]
- 18.Leakey M G, Feibel C S, McDougall I, Walker A. Nature (London) 1995;376:565–571. doi: 10.1038/376565a0. [DOI] [PubMed] [Google Scholar]
- 19.Wood B A. Hominid Cranial Remains, Koobi Fora Research Project. Vol. 4. Oxford: Clarendon; 1991. [Google Scholar]
- 20.Coffing K, Feibel C, Leakey M, Walker A. Am J Phys Anthropol. 1994;93:55–65. doi: 10.1002/ajpa.1330930104. [DOI] [PubMed] [Google Scholar]
- 21.Kay R F. In: Adaptations for Foraging in Nonhuman Primates: Contributions to an Organismal Biology of Prosimians, Monkeys and Apes. Rodman P S, Cant J G H, editors. New York: Columbia Univ. Press; 1984. pp. 21–53. [Google Scholar]
- 22.Ungar P S. Am J Phys Anthropol. 1994;95:197–219. doi: 10.1002/ajpa.1330950207. [DOI] [PubMed] [Google Scholar]
- 23.Ungar P S. Am J Primatol. 1996;38:145–156. doi: 10.1002/(SICI)1098-2345(1996)38:2<145::AID-AJP3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 24.Robinson J T. Mem Transvaal Mus. 1956;9:1–179. [Google Scholar]
- 25.Wolpoff M H. Am J Phys Anthropol. 1973;39:375–394. doi: 10.1002/ajpa.1330390306. [DOI] [PubMed] [Google Scholar]
- 26.Wood B A, Abbott S A. J Anat. 1983;136:197–219. [PMC free article] [PubMed] [Google Scholar]
- 27.McHenry H M. Am J Phys Anthropol. 1984;64:297–306. doi: 10.1002/ajpa.1330640312. [DOI] [PubMed] [Google Scholar]
- 28.Kay R F. Annu Rev Anthropol. 1985;14:315–341. [Google Scholar]
- 29.Suwa G, Wood B A, White T D. Am J Phys Anthropol. 1994;93:407–426. doi: 10.1002/ajpa.1330930402. [DOI] [PubMed] [Google Scholar]
- 30.Grine F E. S Afr J Sci. 1981;77:203–230. [Google Scholar]
- 31.Mahler P E. Ph.D. thesis. Ann Arbor: Univ. of Michigan; 1973. [Google Scholar]
- 32.Alpagut B, Andrews P, Martin L. J Hum Evol. 1990;19:397–422. [Google Scholar]
- 33.Alpagut B, Andrews P, Fortelius M, Kappelman J, Temizsoy I, Çelebi H, Lindsay W. Nature (London) 1996;382:349–351. doi: 10.1038/382349a0. [DOI] [PubMed] [Google Scholar]
- 34.Andrews P. Bull Br Mus (Nat Hist) 1978;30:85–224. [Google Scholar]
- 35.Begun D R, Güleç E. Am J Phys Anthropol. 1998;105:279–314. doi: 10.1002/(SICI)1096-8644(199803)105:3<279::AID-AJPA2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 36.de Bonis L, Melentis J. Cour Forschungsinst Senckenberg. 1984;69:13–23. [Google Scholar]
- 37.Leakey M G, Feibel C S, McDougall I, Ward C, Walker A. Nature (London) 1998;393:62–66. doi: 10.1038/29972. [DOI] [PubMed] [Google Scholar]
- 38.McHenry H M. In: Evolutionary History of the “Robust” Australopithecines. Grine F E, editor. New York: de Gruyter; 1988. pp. 133–147. [Google Scholar]
- 39.Lucas P W, Corlett R T, Luke D A. Z Morphol Anthropol. 1986;76:253–276. [PubMed] [Google Scholar]
- 40.Lucas P W, Teaford M F. In: Colobine Monkeys: Their Ecology, Behaviour and Evolution. Davies A G, Oates J F, editors. Cambridge, U.K.: Cambridge Univ. Press; 1994. pp. 173–203. [Google Scholar]
- 41.Spears I R, Crompton R H. J Hum Evol. 1996;31:517–535. [Google Scholar]
- 42.Strait S G. Evol Anthropol. 1997;5:199–211. [Google Scholar]
- 43.Yamashita N. Am J Phys Anthropol. 1998;106:169–188. doi: 10.1002/(SICI)1096-8644(199806)106:2<169::AID-AJPA5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 44.Meldrum D J, Kay R F. Am J Phys Anthropol. 1997;102:407–428. doi: 10.1002/(SICI)1096-8644(199703)102:3<407::AID-AJPA8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 45.Ungar P S, Kay R F. Proc Natl Acad Sci USA. 1995;92:5479–5481. doi: 10.1073/pnas.92.12.5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lucas P W, Peters C R. In: Development, Function and Evolution of Teeth. Teaford M F, Smith M M, Ferguson M W J, editors. Cambridge, U.K.: Cambridge Univ. Press; 2000. pp. 282–289. [Google Scholar]
- 47.Martin L B. Nature (London) 1985;314:260–263. doi: 10.1038/314260a0. [DOI] [PubMed] [Google Scholar]
- 48.Beynon A D, Wood B A. Am J Phys Anthropol. 1986;70:177–193. doi: 10.1002/ajpa.1330700205. [DOI] [PubMed] [Google Scholar]
- 49.Grine F E, Martin L B. In: Evolutionary History of the “Robust” Australopithecines. Grine F E, editor. New York: de Gruyter; 1988. pp. 3–42. [Google Scholar]
- 50.Beynon A D, Dean M C, Reid D J. Am J Phys Anthropol. 1991;86:295–309. [Google Scholar]
- 51.Macho G A, Thackeray J F. Am J Phys Anthropol. 1992;89:133–143. doi: 10.1002/ajpa.1330890202. [DOI] [PubMed] [Google Scholar]
- 52.Spoor C F, Zonneveld F W, Macho G A. Am J Phys Anthropol. 1993;91:469–484. doi: 10.1002/ajpa.1330910405. [DOI] [PubMed] [Google Scholar]
- 53.Gantt D G. In: Comparative Primate Biology. Volume 1. Systematics, Evolution, and Anatomy. Swindler D R, Erwin J, editors. Vol. 1. New York: Liss; 1986. pp. 453–475. [Google Scholar]
- 54.Andrews P, Martin L. Philos Trans R Soc London B. 1991;334:199–209. doi: 10.1098/rstb.1991.0109. [DOI] [PubMed] [Google Scholar]
- 55.Conroy G C, Pickford M, Senut B, Van Couvering J, Mein P. Nature (London) 1992;356:144–148. doi: 10.1038/356144a0. [DOI] [PubMed] [Google Scholar]
- 56.Martin L B. Ph.D. thesis. London: Univ. of London; 1983. [Google Scholar]
- 57.Maas M C, Dumont E R. Evol Anthropol. 1999;8:133–152. [Google Scholar]
- 58.Kay R F. Am J Phys Anthropol. 1981;55:141–151. doi: 10.1002/ajpa.1330550307. [DOI] [PubMed] [Google Scholar]
- 59.Dumont E R. J Mammal. 1995;76:1127–1136. [Google Scholar]
- 60.Macho G A, Berner M E. Am J Phys Anthropol. 1993;92:189–200. doi: 10.1002/ajpa.1330920208. [DOI] [PubMed] [Google Scholar]
- 61.Teaford M F, Maas M C, Simons E L. Am J Phys Anthropol. 1996;101:527–544. doi: 10.1002/(SICI)1096-8644(199612)101:4<527::AID-AJPA7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 62.Maas M C. Am J Phys Anthropol. 1993;92:217–233. doi: 10.1002/ajpa.1330920210. [DOI] [PubMed] [Google Scholar]
- 63.Maas M C. Am J Phys Anthropol. 1994;95:221–242. doi: 10.1002/ajpa.1330950208. [DOI] [PubMed] [Google Scholar]
- 64.Rensberger J M. In: Tooth Enamel Microstructure. Koenigswald W v, Sander M., editors. Rotterdam: Balkema; 1997. pp. 237–257. [Google Scholar]
- 65.Rensberger J M. In: Development, Function and Evolution of Teeth. Teaford M F, Smith M M, Ferguson M W J, editors. Cambridge, U.K.: Cambridge Univ. Press; 2000. pp. 252–268. [Google Scholar]
- 66.Ungar P S. J Hum Evol. 1996;31:335–366. [Google Scholar]
- 67.Teaford M F, Walker A C. Am J Phys Anthropol. 1984;64:191–200. doi: 10.1002/ajpa.1330640213. [DOI] [PubMed] [Google Scholar]
- 68.Daegling D J, Grine F E. S Afr J Sci. 1994;90:527–532. [Google Scholar]
- 69.Puech P-F, Albertini H. Am J Phys Anthropol. 1984;65:87–91. doi: 10.1002/ajpa.1330650112. [DOI] [PubMed] [Google Scholar]
- 70.Ryan A S, Johanson D C. J Hum Evol. 1989;18:235–268. [Google Scholar]
- 71.Grine F E. J Hum Evol. 1986;15:783–822. [Google Scholar]
- 72.Teaford M F. Scanning Microsc. 1988;2:1149–1166. [PubMed] [Google Scholar]
- 73.Ungar P S. Evol Anthropol. 1998;6:205–217. [Google Scholar]
- 74.Hylander W L. In: Evolutionary History of the “Robust” Australopithecines. Grine F E, editor. New York: de Gruyter; 1988. pp. 55–58. [Google Scholar]
- 75.Daegling D J, Grine F E. Am J Phys Anthropol. 1991;86:321–339. doi: 10.1002/ajpa.1330860302. [DOI] [PubMed] [Google Scholar]
- 76.Smith R J. Ph.D. dissertation. New Haven, CT: Yale Univ.; 1980. [Google Scholar]
- 77.Milton K, Demment M. Am J Primatol. 1988;46:45–52. doi: 10.1002/ajp.1350180105. [DOI] [PubMed] [Google Scholar]
- 78.Milton K. Evol Anthropol. 1999;8:11–21. [Google Scholar]
- 79.Speth J D. J Hum Evol. 1989;18:329–343. [Google Scholar]
- 80.Blumenschine R J, Cavallo J A. Sci Am. 1992;267:90–96. doi: 10.1038/scientificamerican1092-90. [DOI] [PubMed] [Google Scholar]
- 81.de Heinzelin J, Clark J D, White T, Hart W, Renne P, WoldeBabriel G, Beyene Y, Vrba E. Science. 1999;284:625–629. doi: 10.1126/science.284.5414.625. [DOI] [PubMed] [Google Scholar]
- 82.Lee-Thorp J. In: The Evolution of Human Diet. Ungar P S, Teaford M F, editors. New Haven, CT: Greenwood; 2001. , in press. [Google Scholar]
- 83.Bernor R L. In: New Interpretations of Ape and Human Ancestry. Ciochon R L, Corruccini R S, editors. New York: Plenum; 1983. pp. 21–64. [Google Scholar]
- 84.Barry J C. In: Paleoclimate and Evolution, with Emphasis on Human Origins. Vrba E S, Denton G H, Partridge T C, Burckle L H, editors. New Haven, CT: Yale Univ. Press; 1995. pp. 115–134. [Google Scholar]
- 85.Lockwood C A, Kimbel W H, Johanson D C. J Hum Evol. 2000;39:23–55. doi: 10.1006/jhev.2000.0401. [DOI] [PubMed] [Google Scholar]