Abstract
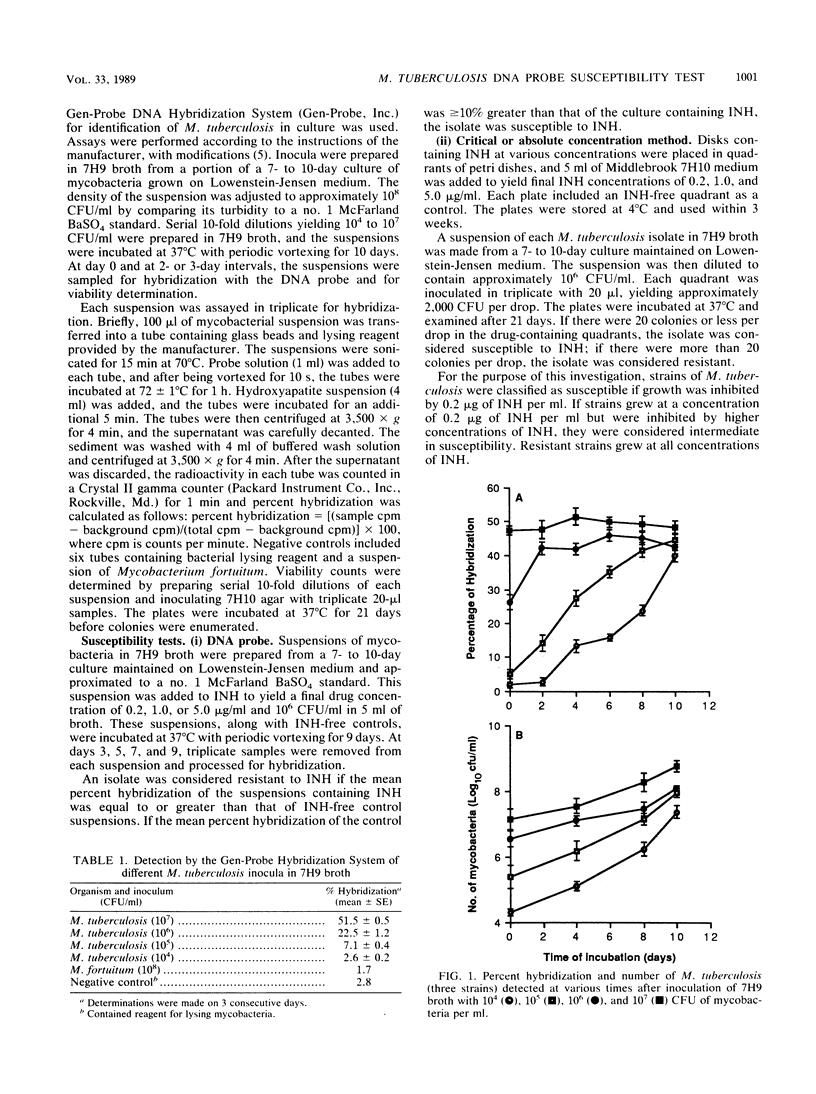
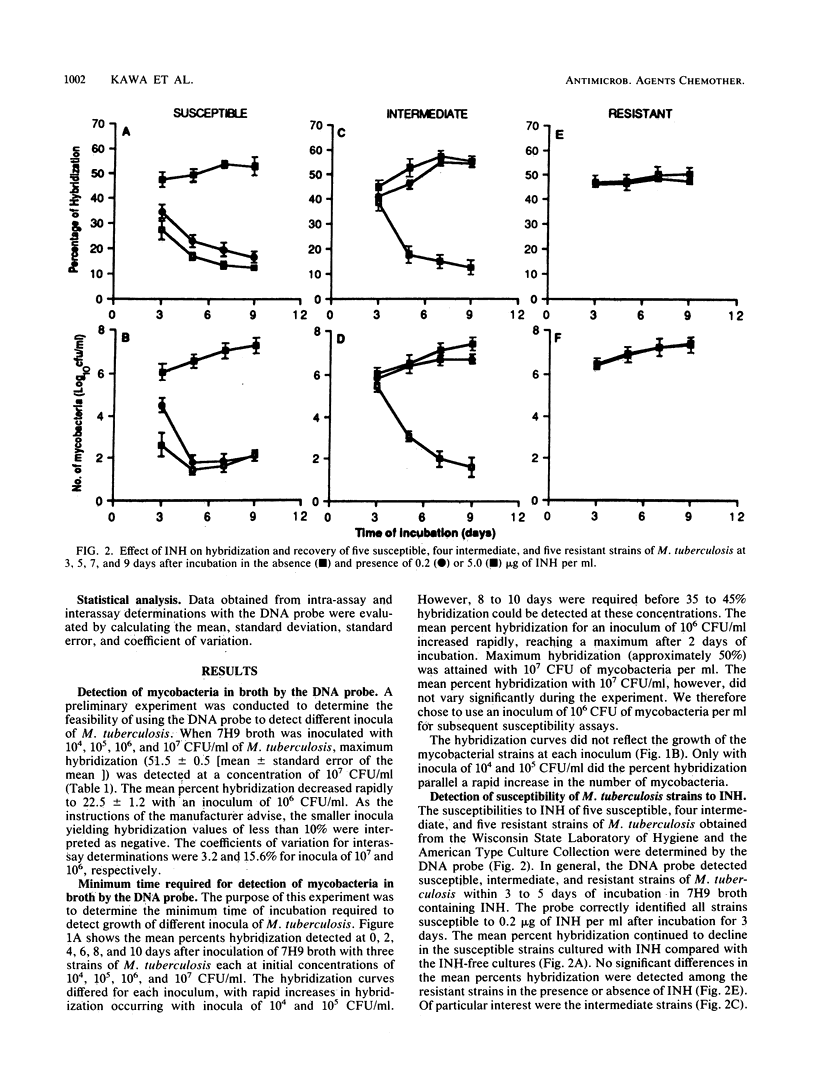
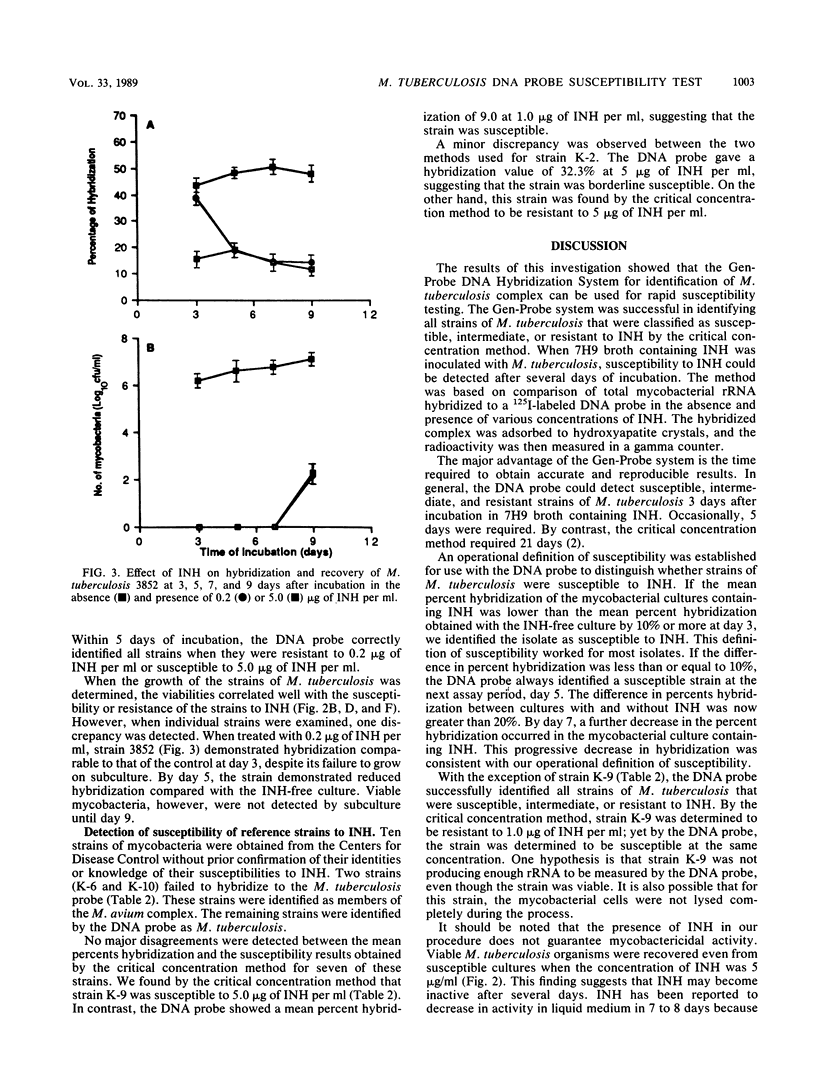
A rapid test was developed for determining the susceptibility of Mycobacterium tuberculosis to isoniazid by using nucleic acid hybridization. The method was based on quantification of total mycobacterial rRNA hybridized to a 125I-labeled DNA probe in the absence and presence of various concentrations of isoniazid. The radioactive hybridized complex was isolated by adsorption to hydroxyapatite crystals and measured in a gamma counter. The susceptibilities of four reference strains and 20 clinical isolates were compared by the Gen-Probe DNA Hybridization System and the critical concentration method. Overall agreement between the two methods was excellent. Results were obtained with the DNA probe after 3 to 5 days of incubation instead of the 14 to 21 days required for the critical concentration method. These findings indicate that susceptibility testing of M. tuberculosis by nucleic acid hybridization has merit for the clinical laboratory. Additional studies are needed to determine the efficacy of the DNA probe method for determining the susceptibility of M. tuberculosis to other antimycobacterial agents and its correlation with clinically significant levels of resistance.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CANETTI G., FROMAN S., GROSSET J., HAUDUROY P., LANGEROVA M., MAHLER H. T., MEISSNER G., MITCHISON D. A., SULA L. MYCOBACTERIA: LABORATORY METHODS FOR TESTING DRUG SENSITIVITY AND RESISTANCE. Bull World Health Organ. 1963;29:565–578. [PMC free article] [PubMed] [Google Scholar]
- David H. L. Probability distribution of drug-resistant mutants in unselected populations of Mycobacterium tuberculosis. Appl Microbiol. 1970 Nov;20(5):810–814. doi: 10.1128/am.20.5.810-814.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake T. A., Hindler J. A., Berlin O. G., Bruckner D. A. Rapid identification of Mycobacterium avium complex in culture using DNA probes. J Clin Microbiol. 1987 Aug;25(8):1442–1445. doi: 10.1128/jcm.25.8.1442-1445.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner P. D., Kiehn T. E., Cammarata R., Hosmer M. Rapid detection and identification of pathogenic mycobacteria by combining radiometric and nucleic acid probe methods. J Clin Microbiol. 1988 Jul;26(7):1349–1352. doi: 10.1128/jcm.26.7.1349-1352.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GANGADHARAM P. R., HAROLD F. M., SCHAEFER W. B. Selective inhibition of nucleic acid synthesis in Mycobacterium tuberculosis by isoniazid. Nature. 1963 May 18;198:712–714. doi: 10.1038/198712b0. [DOI] [PubMed] [Google Scholar]
- Gonzalez R., Hanna B. A. Evaluation of Gen-Probe DNA hybridization systems for the identification of Mycobacterium tuberculosis and Mycobacterium avium-intracellulare. Diagn Microbiol Infect Dis. 1987 Oct;8(2):69–77. doi: 10.1016/0732-8893(87)90152-0. [DOI] [PubMed] [Google Scholar]
- Heifets L. B., Iseman M. D., Cook J. L., Lindholm-Levy P. J., Drupa I. Determination of in vitro susceptibility of Mycobacterium tuberculosis to cephalosporins by radiometric and conventional methods. Antimicrob Agents Chemother. 1985 Jan;27(1):11–15. doi: 10.1128/aac.27.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertcher J. A., Chen M. F., Charache P., Hwangbo C. C., Camargo E. E., McIntyre P. A., Wagner H. N., Jr Rapid radiometric susceptibility testing of Mycobacterium tuberculosis. Am Rev Respir Dis. 1978 Apr;117(4):631–637. doi: 10.1164/arrd.1978.117.4.631. [DOI] [PubMed] [Google Scholar]
- Lee C. N., Heifets L. B. Determination of minimal inhibitory concentrations of antituberculosis drugs by radiometric and conventional methods. Am Rev Respir Dis. 1987 Aug;136(2):349–352. doi: 10.1164/ajrccm/136.2.349. [DOI] [PubMed] [Google Scholar]
- Musial C. E., Tice L. S., Stockman L., Roberts G. D. Identification of mycobacteria from culture by using the Gen-Probe Rapid Diagnostic System for Mycobacterium avium complex and Mycobacterium tuberculosis complex. J Clin Microbiol. 1988 Oct;26(10):2120–2123. doi: 10.1128/jcm.26.10.2120-2123.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L. E., Hoffner S. E., Anséhn S. Rapid susceptibility testing of Mycobacterium tuberculosis by bioluminescence assay of mycobacterial ATP. Antimicrob Agents Chemother. 1988 Aug;32(8):1208–1212. doi: 10.1128/aac.32.8.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G. D., Goodman N. L., Heifets L., Larsh H. W., Lindner T. H., McClatchy J. K., McGinnis M. R., Siddiqi S. H., Wright P. Evaluation of the BACTEC radiometric method for recovery of mycobacteria and drug susceptibility testing of Mycobacterium tuberculosis from acid-fast smear-positive specimens. J Clin Microbiol. 1983 Sep;18(3):689–696. doi: 10.1128/jcm.18.3.689-696.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi S. H., Hawkins J. E., Laszlo A. Interlaboratory drug susceptibility testing of Mycobacterium tuberculosis by a radiometric procedure and two conventional methods. J Clin Microbiol. 1985 Dec;22(6):919–923. doi: 10.1128/jcm.22.6.919-923.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider D. E., Jr, Good R. C., Kilburn J. O., Laskowski L. F., Jr, Lusk R. H., Marr J. J., Reggiardo Z., Middlebrook G. Rapid drug-susceptibility testing of Mycobacterium tuberculosis. Am Rev Respir Dis. 1981 Apr;123(4 Pt 1):402–406. doi: 10.1164/arrd.1981.123.4.402. [DOI] [PubMed] [Google Scholar]
- Vannier A. M., Tarrand J. J., Murray P. R. Mycobacterial cross contamination during radiometric culturing. J Clin Microbiol. 1988 Sep;26(9):1867–1868. doi: 10.1128/jcm.26.9.1867-1868.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimpenny J. W. Effect of isoniazid on biosynthesis in Mycobacterium tuberculosis var. bovis BCG. J Gen Microbiol. 1967 Jun;47(3):379–388. doi: 10.1099/00221287-47-3-379. [DOI] [PubMed] [Google Scholar]
- Youatt J. A review of the action of isoniazid. Am Rev Respir Dis. 1969 May;99(5):729–749. doi: 10.1164/arrd.1969.99.5.729. [DOI] [PubMed] [Google Scholar]


