Abstract
Gap junctions, composed of proteins from the connexin family, allow for intercellular communication between cells in tissues and are important in development, tissue/cellular homeostasis and carcinogenesis. Genome databases indicate that there are at least 20 connexins in the mouse and human. Connexin phosphorylation has been implicated in connexin assembly into gap junctions, gap junction turnover and cell signaling events that occur in response to tumor promoters and oncogenes. Connexin43 (Cx43), the most widely expressed and abundant gap junction protein, can be phosphorylated at several different serine and tyrosine residues. Here, we focus on the dynamic regulation of Cx43 phosphorylation in tissue and how these regulatory events are affected during development, wound healing and carcinogenesis. Activation of several kinases including protein kinase A, protein kinase C, p34cdc2/cyclin B kinase, casein kinase 1, mitogen-activated protein kinase and pp60src kinase can lead to phosphorylation of different residues in the C-terminal region of Cx43. The use of antibodies specific for phosphorylation at defined residues has allowed examination of specific phosphorylation events both in tissue culture and in vivo. These new antibody tools and those under development will allow us to correlate specific phosphorylation events with changes in connexin function.
Keywords: Connexin, Gap Junction, Phosphorylation, Phosphospecific antibodies, Cell Signaling
1. Introduction
Gap junctions are tightly packed clusters of intercellular channels that directly connect the cytoplasms of adjacent cells. They coordinate cell-to-cell communication within tissues and allow for the transfer of molecules less than 1000 Daltons between cells such as ions, simple sugars, amino acids, nucleotides and second messengers (e.g., Ca2+,cAMP, cGMP, IP3) [1-3]. In vertebrates, gap junctions are composed of proteins from the connexin family, which is comprised of 21 members in humans [1-3]. Connexins are commonly designated with numerical suffixes referring to the molecular weight of the deduced sequence in kilodaltons (e.g., connexin43 or Cx43). Connexin proteins possess four hydrophobic membrane-spanning domains. Two conserved, extracellular domains are involved in paired hemichannel docking with counterparts from the adjacent membrane leaving three cytoplasmic domains corresponding to the amino-terminal region, a loop between transmembrane domains 2 and 3, and the carboxyterminal tail region. Connexins are differentially expressed in tissues with some being significantly expressed in only a few tissues and some, like Cx43, being more widespread [2, 3]. Gap junctions play significant regulatory roles in embryonic development, electrical coupling, metabolic transport, apoptosis, differentiation, tissue homeostasis and carcinogenesis [1, 2, 4].
Important insights into these roles have been obtained in recent years from disease-related deficiencies in humans and targeted disruption experiments in mice [2, 3, 5, 6]. Genetic linkage analysis has implicated Cx26 mutations as the major cause of non-syndromic hereditary deafness in humans [7]. Mutations in Cx50 have been implicated in the development of certain hereditary cataracts in humans [8, 9]. Mutations within the Cx32 gene are associated with the X-linked peripheral nerve disorder Charcot-Marie Tooth Syndrome [10, 11] and Cx32 deficient mice are several-fold more susceptible to induction of liver [12-14] and lung [15] cancer. Cx43 mutations cause the pleiotropic phenotype of oculodentodigital dysplasia [16]. Female Cx37 deficient mice fail to ovulate and are infertile [17]. Cx26 and Cx45 deficient mice die in utero and Cx43 deficient mice die perinatally due to heart malformations [18]. Cx43(±) and Cx40(−−) mice have heart conduction abnormalities [19, 20]. These disparate phenotypes not only show the diversity of the expression pattern of connexins, but they also illustrate that gap junctions play different roles in different tissues. Mouse “knock in” experiments, in which one connexin isotype is replaced with another, have shown that individual connexins play distinct roles in specific cellular processes. For example, ablation of either the lens fiber cell connexins Cx46 or Cx50 led to cataract formation while lens growth was only impaired in the Cx50-/-mice [21, 22]. Interestingly, targeted replacement of Cx50 with Cx46 corrected defects in differentiation and prevented cataract formation but did not restore growth control [23]. In other mouse studies, when Cx43 was replaced by Cx32 or Cx40, the mice survived past birth but the developmental defects were only moderated by replacement with Cx32 and several additional defects became apparent in both groups of mice [24]. Replacement of Cx43 with a truncated version (Cx43K258Stop) lacking the C-terminal, cytoplasmic tail region yielded mice that died shortly after birth due to an epidermal barrier defect, not the heart defect that is present in Cx43 deficient mice [25]. The C-terminal region has been shown to have multiple sites of phosphorylation and protein interaction and hence is likely involved in Cx43 regulation.
Connexin phosphorylation has been reviewed several times recently [26-31]. Connexin phosphorylation has been correlated with changes in gap junction assembly, stability and channel properties [26-31]. Connexins of the 3 major evolutionary groups (α, β, and γ) can be phosphorylated, and, given that tissues can express multiple connexins, it seems likely that a single cell could express multiple phosphorylated connexins. However, no “consensus” connexin phosphorylation sequences between the different family members have been identified. While most of the research has been performed in mammalian systems, both chick and fish connexins have been shown to be phosphorylated [32, 33]. Up until recently, essentially all of this research was based on cell culture models. This review concentrates on the presence of Cx43 phosphorylation in tissues and offers speculation on the role that these events play in tissue function. These studies are possible because phosphorylation-state specific antibodies have become available for several phosphorylation sites in Cx43.
2. Phosphorylation of Cx43
Several reports have shown that Cx43 has a half-life in the range of 1-3 h in cultured cells or in tissues [34-38]. A fast turnover rate could imply a high level of post-translational regulation. Indeed, the pioneering work of Musil and Good enough, the Lau laboratory and several other investigators have shown that Cx43 is differentially phosphorylated throughout its life cycle in homeostatic cells [34-36, 39-43]. Cx43 demonstrates multiple electrophoretic isoforms when analyzed by polyacrylamide gel electrophoresis (SDS-PAGE), including a faster migrating form that includes non-phosphorylated (P0 or NP) Cx43, and at least two slower migrating forms, commonly termed P1 and P2. Both P1 and P2 co-migrate with P0 following alkaline phosphatase treatment, suggesting that phosphorylation is the primary covalent modification detected in SDS-PAGE analysis [35, 42]. However, we and others have found that phosphorylated species can migrate with the P0 band in SDS-PAGE [44]. Phosphoamino acid analysis indicates the majority of the phosphorylation events occur on serines [42, 45-47], although tyrosine phosphorylation has been observed in the presence of activated pp60src [35, 48]. The C-terminal region of Cx43 appears to be the primary region that becomes phosphorylated, but Cx56 can be phosphorylated within the cytoplasmic loop region, in addition to its C-terminal domain [49]. Cx43 does not contain serine residues in its intracellular loop region, and no reports of phosphorylation of the N-terminal region of connexins have been presented.
PKC activators [e.g., the phorbol ester TPA (12-O-tetradeconylphorbol-13-acetate also known as PMA)] both increase Cx43 phosphorylation and decrease gap junction communication in a number of different cell types [37, 39, 40, 50, 51]. TPA treatment has been shown to reduce the permeability of Cx43 channels via phosphorylation of serine 368 (S368) [52] and dramatically decrease gap junction assembly [37]. PKC has been shown to phosphorylate Cx43 at S368 and S372 [52-54] in vitro. Phosphorylation of S262 and S368 has been shown to be increased in response to TPA. The former has been linked to increased cellular proliferation through an unknown mechanism [55] while the latter has been shown to underlie the TPA-induced reduction in intercellular communication and alteration of single channel behavior [52]. PKCα and ε were found to associate with Cx43 in cardiomyocytes [56]. Fibroblast growth factor-2, which decreases cardiomyocyte gap junctional permeability and increases Cx43 phosphorylation, increased colocalization of PKCε with Cx43 [57].
Casein kinase 1, particularly the δ isoform, has been shown to interact with and phosphorylate Cx43 on serine(s) 325, 328 or 330 in vitro [58]. Cx43 may also be a direct substrate for casein kinase 1 in vivo as these residues are major phosphorylation sites of cellular Cx43 [58]. Certainly other kinases phosphorylate Cx43. Several residues in the tandem serine repeat region can be phosphorylated (i.e., serines 365, 368, 369 and 373) in response to follicle-stimulating hormone, probably at least partially through a PKA-mediated mechanism [59]. There is experimental evidence that Cx43 can be phosphorylated on at least 12 of the 21 serines [26] and 2 of the tyrosines (when src kinase is active [60]) in the cytoplasmic tail region (amino acids 250-382). Certainly, it is possible that multiple kinases may phosphorylate the same residue. For example, serine 255 can be phosphorylated by both MAPK and cdc2 [47, 61]. Thus, considerable evidence indicates that Cx43 is a highly phosphorylated and a highly regulated protein.
3. Phosphospecific Cx43 antibodies
The advent of phosphorylation specific antibodies has revolutionized the study of cell signaling pathways with the availability of hundreds of antibodies reported to be specific for a certain phosphorylation event on the protein of interest. For example, phosphorylation specific antibodies exist for HER-2/neu, epidermal growth factor receptor, insulin-like growth factor receptor, PKC isoforms, STAT family members, β-catenin, Smad family members, p53, Rb and many others. In cases like the ERK/MAP kinase signaling pathway, a whole series of phosphospecific antibodies can be used to follow up- and downstream phosphorylation events on c-Raf, MEK1/2 and ERK1/2. These antibodies are now being used clinically for a variety of purposes that include Alzheimer’s disease, Parkinsonism, and cancer detection/diagnosis.
Two commercially available phosphospecific antibodies for Cx43 have been shown to be specific, one at S368 (pS368) [44] and another at S262 (pS262) [55]. S368 is the primary Cx43 substrate for PKC following activation by TPA treatment [52] and the pS368 antibody binding increases ∼8 fold upon TPA treatment of HeLa cells expressing exogenous Cx43. Comparison of wild-type and S368A mutant Cx43 transfected into fibroblasts obtained from Cx43 deficient mice showed that phosphorylation at S368 results in a reduction in unitary channel conductance of the gap junctions (50 pS channels are favored over 100 pS channels). The pS368 antibody was also used to show that phosphorylation on S368 increased in S and G2/M phases of the cell cycle [44]. Mitogenic stimulation of cardiomyocytes has also been associated with decreased GJC and PKC-mediated phosphorylation on S262 [55]. Over-expression of exogenous Cx43 or a S262A mutant led to a decrease in the number of cells entering S phase. However, a S262D mutant, which mimics the phosphorylated charge, did not affect the number of cells entering S phase indicating that phosphorylation on this site may play a role in a G1/S checkpoint. A commercial monoclonal antibody (Zymed/Invitrogen 13-8300) prepared to the C-terminal region has been shown to bind to an epitope near the C-terminus only when it is not phosphorylated [62], although others have shown that this antibody can bind to Cx43 phosphorylated upon TPA treatment, presumably at different sites [63]. A few other commercial phosphorylation site-specific antibodies are available but their specificity remains to be demonstrated. Individual investigators have also prepared antibodies for Cx43 specific for phosphorylation at S279/282 [64] and S325/328/330 (discussed below). The pS279/282 antibody was used to show that epidermal growth factor dramatically increased phosphorylation of these residues both at apparent gap junctions and in cytoplasmic membranes [64]. Thus, a panel of Cx43 phosphospecific antibodies is developing that will help elucidate mechanisms of gap junction regulation.
4. Cx43 Phosphorylation in Tissues
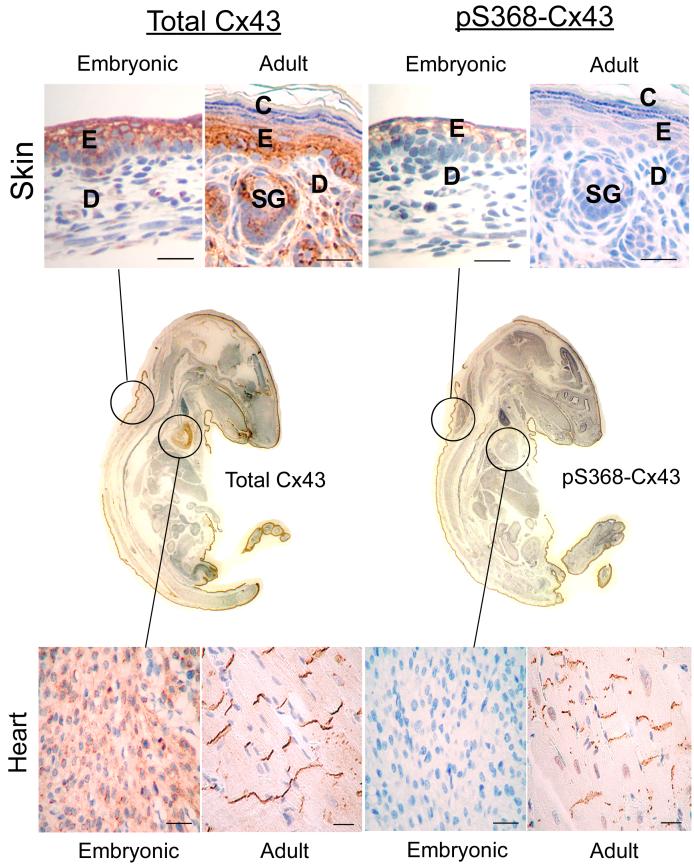
As indicated above, the mobility of Cx43 in SDS-PAGE can shift upon phosphorylation although the residues responsible for the shift are unknown and phosphorylation of some residues does not appear to affect the shift. Nonetheless, several studies described below have shown apparent changes in phosphorylation of Cx43 in tissue based on immunoblot analysis. Probing tissue with phosphospecific antibodies has many advantages. The reagents are specific for a single phosphorylation event and hence relate the phosphorylation status whether or not the SDS-PAGE mobility shifts. They can be used in immunohistochemical assays to localize changes in tissues to indicate the importance of these regulatory events in vivo. Finally, given the likelihood that more than a dozen phosphorylation sites exist in Cx43, the use of combinations of these antibodies and knowledge of the kinase systems responsible for phosphorylation at these sites could likely give a snapshot of the signaling pathways affecting gap junction regulation at a particular time point. As an example, we have utilized the pS368 antibody to examine phosphorylation at this residue in whole mounts of embryonic 14.5 day mice and compared that with adult mice (12 months). We observed significant tissue-specific changes in the level of pS368 during development. pS368 levels changed from high to low in the skin and cornea and conversely from low to high in the heart (Fig. 1 & Table 1).
Fig. 1.
Immunohistochemical detection of paraffin-embedded adult (12 months) and embryonic (E14.5) mouse skin and heart tissue using antibodies to total Cx43 (left column) and Cx43 phosphorylated on ser368 (pS368-Cx43, right column). Center panels: IHC detection of total Cx43 (left) and pS368-Cx43 (right) in paraffin-embedded whole neonatal mouse. E=epidermis, D=dermis, SG=sweat gland, C=cornified stratus. Bar=25 μm.
Table
| Table 1 | pS368-Cx43 | |
|---|---|---|
| Tissue | Embryonic | Adult |
| Heart | +/- | +++ |
| Skin | ++ | +/- |
| Cornea | ++ | - |
| Lens | - | - |
4.1. Role of Cx43 Phosphorylation in Heart
Several connexins are expressed in heart tissue including Cx40, Cx45 and Cx43 [2, 3]. Gap junction communication allows for synchronized signal transmission during myocardial contraction [65]. Recently, several studies have investigated the possibility of dysfunctional gap junction communication and Cx43 mutation in human cardiac syndromes. One study linked Cx43 mutations with visceroatrial heterotaxy in children [66]; however, other groups were unable to recapitulate these findings [67, 68]. Yet another study identified substitution mutations within Cx43 that correlated with hypoplastic left heart syndrome suggestive of possible Cx43 pseudogene recombination involvement [69].
In rodents, several groups have demonstrated the importance of connexins in cardiac function using genetically-engineered mouse models. Cx40-deficient mice exhibit arrhythmias [70, 71]. However, their intensity is mouse strain sensitive suggesting modifier gene influence [72]. Cx43-deficient embryos (E15.5) demonstrate altered conduction velocity [73]. To avoid neonate lethality due to left ventricular outflow tract occlusion [18], cardiomyocyte-specific Cx43-deficient mice were created by several laboratories using CRE-lox technology. In one study, these animals exhibited arrhythmias and survived for months [74]. In contrast, a second group demonstrated early neonate lethality associated with a variety of cardiac abnormalities [75]. While strain background may play a role in the differing severity of effects, both studies underline the importance of Cx43 in normal mouse heart function.
Studies investigating Cx43 phosphorylation during ischemia have yielded interesting correlations between phospho-Cx43 status and the activity of several kinases. In rodent models, ischemia results in decreased Cx43 phosphorylation as determined by increased migration (i.e., decreased phosphorylation at unspecified phosphorylation sites) of Cx43 in SDS-PAGE analysis [76, 77]. This decrease in phosphorylation is presumably due to altered kinase or phosphatase activity [78]. Preconditioning of the hearts prior to ischemic insult leads to decreased damage, preserves Cx43 migration [76, 77, 79] and results in the co-localization of Cx43 with several kinases including PKC and p38MAPK [79]. These results clearly demonstrate that Cx43 is important in normal cardiac function and that the highly dynamic phosphorylation of Cx43 plays an integral regulatory role in cardiac function.
Few studies have evaluated the role of Cx43 phosphorylation during cardiac development [80]. We analyzed embryonic and adult mouse heart tissue for total Cx43 as well as Cx43 phosphorylated on S368 (Fig. 1). Clearly even at this embryonic stage (E14.5), Cx43 is highly expressed in the heart and continues to be expressed in adult heart tissue. Strikingly, pS368 reactivity is nearly non-existent in embryonic heart but is dramatically present at high levels in adult tissue. Developmental regulation of specific kinase activities that directly or indirectly lead to Cx43 phosphorylation may explain these results [80]. It is interesting to contrast this change from low pS368 levels in embryonic heart to high levels in adult heart to the distinct pattern seen in a different tissue type such as skin or cornea where there is a higher level of pS368 in embryonic tissue that is subsequently decreased in adults (Table 1 and below).
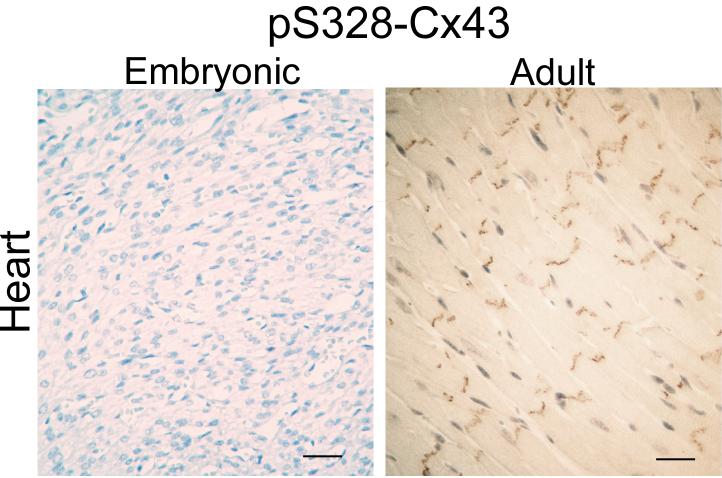
Similarly, we analyzed embryonic and adult mouse heart tissue using immunohistochemical techniques with a phosphospecific antibody reactive against 3 serines (S325, S328, S330) on Cx43. These serines were previously shown to be potential substrates for casein kinase 1 and to be involved in gap junction assembly [58]. Here, similar to pS368 signal, we detected minimal reactivity in embryonic tissue in contrast to adult tissue where high levels of phosphorylation were evident (Fig. 2). Although phosphorylation of these sites appears to be involved in normal Cx43 processing and gap junction assembly, clearly at this stage of development where total Cx43 is not completely organized at the intercalated disk, the majority of Cx43 is not phosphorylated at these sites.
Fig. 2.
Immunohistochemical detection of paraffin-embedded adult (12 months) and embryonic (E14.5) heart tissue using antibodies to total Cx43 (left column) and Cx43 phosphorylated on ser328 (pS328-Cx43, right column). Bar=25 μm.
4.2. Eye
Phosphorylation of Cx43 on Serine368 in Embryonic and Adult Mouse Eye
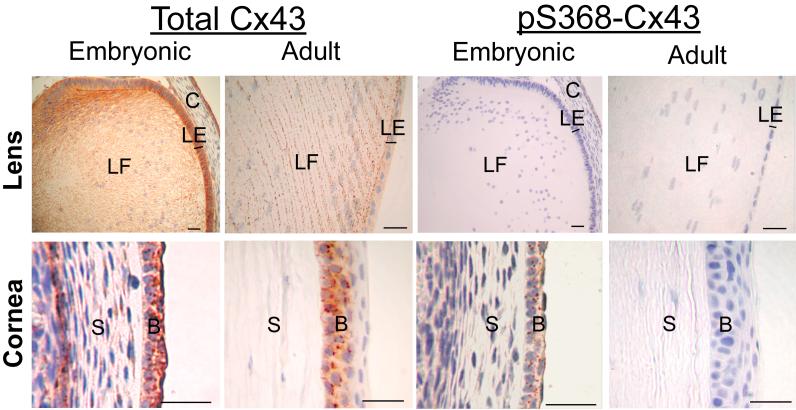
The eye is comprised of several distinct tissue types including the retina (neural and pigmented), cornea and lens. Several connexin types are expressed in both human and mouse ocular tissue including Cx46, Cx50 and Cx43 [2, 3, 23]. In the lens fiber cell, Cx46 and Cx50 are highly expressed presumably due to their role in nutrient supply and waste removal throughout this a vascular tissue. Both Cx46 and Cx50 are phosphoproteins and PKC and other kinase systems have been implicated in cultured lens cells [32, 49, 81] but no data is available for intact tissue. Cx43 is also expressed in cornea, lens and retina but again there have been no reports investigating Cx43 phosphorylation patterns in any portion of the eye. In support of previous studies, Cx43 expression can be detected in formalin-fixed, paraffin-embedded adult (12 month) and embryonic (E14.5) lens and cornea (Fig. 3). Interestingly, the level of phosphorylation at S368-Cx43 appears to be developmentally regulated with distinct differences in embryonic and adult cornea. In the lens, both embryonic and adult tissue exhibit high levels of Cx43 protein with an absence of detectable pS368-Cx43 signal (Fig. 3). Clearly, the distribution of Cx43 seen in the embryonic lens changes as the lens fibers elongate and differentiate into adult tissue. Therefore, embryonic and adult tissue both exhibit high levels of Cx43 with an absence of significant pS368 modification. The embryonic tissue that will differentiate into adult cornea demonstrates high levels of Cx43 and significant pS368 (Fig. 3). However, in contrast to embryonic tissue, the adult cornea maintains a high level of total Cx43 but contains very little detectable pS368 phosphoform (Fig. 3). One could hypothesize that pS368 phosphorylation may allow the precursor corneal tissue to respond/prevent signals that aid/inhibit differentiation of this tissue type into adult cornea.
Fig. 3.
Immunohistochemical detection of paraffin-embedded adult (12 months) and embryonic (E14.5) mouse lens and corneal tissue using antibodies to total Cx43 (left column) and Cx43 phosphorylated on ser368 (pS368-Cx43, right column). LF=lens fiber, LE=lens epithelia, C=precursor cornea, S=stroma, B=basal epithelia. Bar=25 μm.
4.3. Skin
Mutations in several connexin genes have recently been associated with erythrokeratodermia variabilis, an inherited human skin disorder [82, 83]. Numerous studies have demonstrated that human and rodent skin express multiple connexins. In particular, Cx43 and Cx26 protein levels are high in human and rodent skin and display very specific localization. In mouse epidermis, Cx43 protein is localized in the less differentiated, basal keratinocyte cell layer with Cx26 protein localized in more differentiated, upper spinous and granular layers [84-86]. In contrast, human epidermis exhibits a different connexin localization with Cx43 protein primarily in the upper, differentiated spinous and granulosa layers whereas Cx26 is expressed at much lower levels in keratinocytes but is abundantly expressed in hair and eccrine sweat glands [87-89]. This species-specific variation in Cx26 and Cx43 localization in the epidermis remains unexplained.
Few studies have described altered phosphorylation during normal development or differentiation in skin. Antibodies reactive against total Cx43 and specific for pS368-Cx43 were used to investigate expression/localization in adult mouse skin and to compare embryonic to adult Cx43 total expression and phosphorylation patterns. As shown in Fig. 1, Cx43 expression was similar to that previously observed in adult mouse skin [84-86] with the majority of Cx43 in basal and spinous layers and minimal reactivity in granulosa layers. Likewise, pS368 levels are moderate in adult skin and display patterns similar to that seen for total Cx43 (Fig. 1). In contrast, evaluation of embryonic mouse skin (E14.5, Fig. 1) revealed much higher levels of pS368 demonstrating developmental control of this Cx43 post-translational modification. This regulation may or may not be completely linked to differentiation status as the skin begins to stratify into the multi-layered adult tissue. The total level of Cx43 in embryonic skin was also high and demonstrated patterns of expression similar to adult skin. It is interesting to contrast this pattern of diminishing pS368 seen in maturing skin with the dramatic increase in pS368 observed when embryonic and adult heart tissue are compared (Table 1).
5. Dynamic changes in Cx43 phosphorylation in tissues
Human Skin Wounds:
Expression of connexin genes is tissue-specific and although several connexins can be found in skin, Cx43 is the predominant connexin in human epidermis and in cultures of human keratinocytes [89, 90]. Keratinocyte proliferation occurs in the basal layer of the epidermis with keratinocytes undergoing terminal differentiation as they migrate through the suprabasal, spinous and granular layers to the skin surface. Connexin proteins are differentially expressed in skin with lower expression in the proliferative regions and increased expression upon differentiation (Fig. 4) [90-94].
Fig. 4.
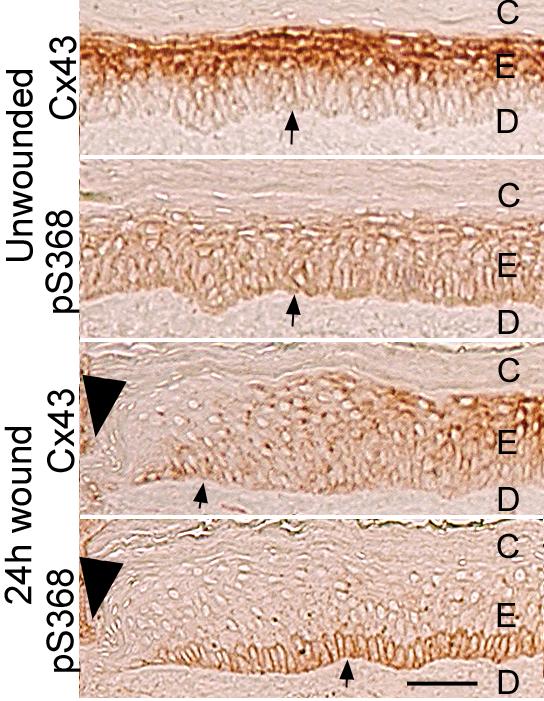
Immunohistochemistry detection of normal and wounded human skin for total Cx43 and pS368. Serial sections of unwounded human skin and 24 h wounded tissue were labeled with antibodies to either Cx43 or pS368 as indicated. Dead cornified cells (marked as C in uppermost layers) or dermal cells (marked D) lack Cx43 staining. Tissue regions are indicated as follows: epidermis=E; dermis=D; cornified layer=C; basement membrane; arrow, wound edge; arrowhead. Bar = 50μm.
Wounding of the epidermis activates cell migration across the wound bed, increases proliferation, and promotes changes in cell communication [94-99]. It has been suggested that gap junctional intercellular communication may regulate certain aspects of the wound healing process including initiation/synchronization of cellular migration [93, 94, 98]. Following wounding, connexin expression is decreased at the wound edge but expression is enhanced at unwounded adjacent areas and upon wound closure when the cells differentiate [93, 98, 100]. Application of Cx43 antisense to wounds accelerated keratinocyte migration and the rate of wound repair resulting in less scaring [101]. Closure of wounds was one day faster in Cx43-deficient mice [94]. These results indicate that Cx43 regulation plays an important role in wound repair. Other connexins may play similar roles in wound healing in disparate tissues. For example, Cx32 expression levels decrease post-hepatectomy in mice with restoration to normal levels following completion of regeneration [102].
Examination of pS368 levels in normal human skin tissue using the phosphosite-specific antibody showed relatively even distribution throughout the epidermal layers ([103] and Fig. 4). However, 24h after wounding (and not at 6 or 72h), pS368 levels were dramatically increased in basal keratinocytes and essentially lost from suprabasal layers adjacent to the wound (i.e., within 200 μm)(Fig. 4). Scratch wounding of primary human keratinocytes caused a PKC-dependent increase in pS368 in cells adjacent to the scratch with a time course similar to tissue wounding. Keratinocytes at the edge of the scratch also transferred dye much less efficiently at 24h in a manner dependent on PKC [103]. However, keratinocyte migration to fill the scratch required early (<6h) gap junctional communication. Thus, the available evidence indicates that PKC-dependent phosphorylation of Cx43 at S368 creates dynamic communication compartments that can temporally and spatially regulate wound healing.
Altered Cx43 Phosphorylation During Mouse Skin Carcinogenesis:
Several studies employing multi-stage mouse skin tumorigenesis protocols using 7,12-dimethylbenzanthracene (DMBA) or N-methyl-N’-nitro-guanidine as initiators and TPA as a tumor promoter have demonstrated alterations in Cx43 and Cx26 mRNA and protein levels as well as aberrant localization [84-86]. Early in the skin carcinogenesis process, hyperplastic skin shows little change in Cx43 and Cx26 protein levels. However, two studies reported increased Cx43 and Cx26 protein levels in papillomas [84, 86] associated with abnormal co-localization of Cx26 and Cx43. In contrast, both Cx43 and Cx26 message (via in situ hybridization [84]) and/or protein levels (via immunohistochemistry and Western [84-86]) are dramatically decreased in squamous cell carcinomas. One of these studies also demonstrated decreased Cx43 protein levels in lymph node-associated tumor metastases [85]. These studies suggest that although initially Cx43 levels can be increased in papillomas, the protein is often mislocalized and skin tumor progression to squamous cell carcinoma and metastasis involves further loss of functional Cx43 through reduced Cx43 expression and mislocalization. In further linkage of function to expression levels, decreased gap junctional communication was observed in cell lines representing progressively more advanced skin tumor types [104].
Many of these studies clearly implicate Cx43 as a tumor suppressor in rodent skin. However, analysis of genetically engineered mice heterozygous for Cx43 revealed no increase in skin tumor incidence or multiplicity when exposed to DMBA and TPA (multi-stage carcinogenesis protocol) [105]. Analysis of resulting skin tumors in this study revealed that one Cx43 wild-type allele remained suggesting that complete loss of Cx43 is not necessary for skin tumor formation. It remains to be seen if epidermal-specific ablation of Cx43 in a targeted genetically engineered mouse model will confer increased susceptibility to skin carcinogenesis. As mentioned, previous studies have also demonstrated altered Cx26 levels/function suggesting that loss of Cx43 may not be sufficient to induce skin carcinogenesis and/or that there is a redundant function of Cx43 and Cx26 in skin tumor suppression. While there have been no reports linking connexin mutations in human skin with neoplasia, Tada et al. reported detection of poorly developed gap junctions and increased cytoplasmic Cx43 in human basal cell carcinomas and squamous cell carcinomas via ultrastructural localization techniques suggesting the possibility of reduced gap junctional function possibly resulting from abnormal processing, gating or stability of the channels [106].
The association between differentiation, proliferation and connexin expression is supported by many interesting studies. One study that compared connexin levels in primary human keratinocyte strains and a human squamous cell carcinoma cell line found that higher Cx43 and Cx26 levels correlated with higher proliferative status in contrast to lower Cx43/Cx26 and higher Cx31/Cx31.1 protein levels in post-confluent cells [107]. These results clearly contrast with observations using intact human skin where Cx43 expression is limited to differentiated keratinocyte cell layers [90]. This study suggests that connexin type expression is tightly associated with the proliferative and/or differentiation status in keratinocytes. This is further supported by a report utilizing keratinocyte cultures where decreased Cx43/Cx26 levels and increased Cx30 and Cx31.1 levels were associated with calcium-induced differentiation [108].
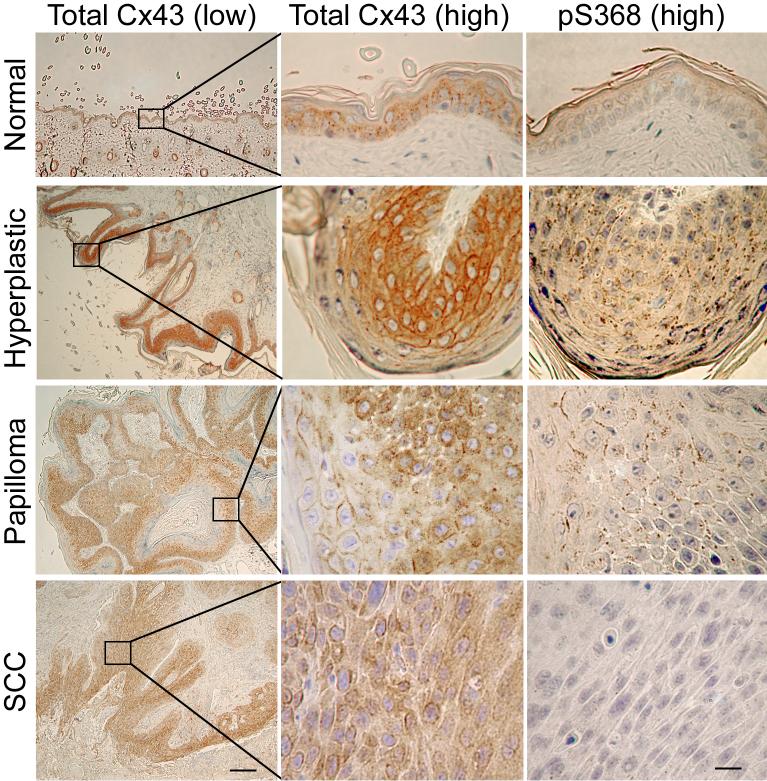
To date few published studies have addressed alterations in Cx43 phosphorylation during skin carcinogenesis. Immunohistochemical investigation of mouse skin neoplasia for total Cx43 and Cx43 phosphorylated at S368 in DMBA/TPA-induced mouse skin showed dramatic changes during progression (Fig. 5). An increase in total Cx43 reactivity was observed in hyperplastic skin compared to normal skin with subsequent decreased levels and nonfunctional, cytoplasmic localization in more aggressive tumors (papilloma vs. squamous cell carcinoma) consistent with previously reported studies [84-86]. An increase in pS368 immuno-reactivity in hyperplastic skin and papillomas was observed compared to adjacent normal tissue. Furthermore, a reversal of this trend was observed with invasive tumors (squamous cell carcinoma-SCC) which demonstrated dramatically decreased pS368 levels compared to papillomas. These results suggest that even though Cx43 protein is not decreased at early stages of the skin carcinogenesis process, gap junctional communication may be down-regulated via increased PKC-mediated phosphorylation of S368. As tumors become invasive, Cx43 levels are reduced, the protein is not localized to junctions and pS368 levels decrease.
Fig. 5.
Immunohistochemical detection of paraffin-embedded of DMBA/TPA treated adult mouse skin using antibodies to total Cx43 (1st low magnification and 2nd higher magnification columns) and Cx43 phosphorylated on ser368 (pS368-Cx43, 3rd column). Normal skin (1st row), hyperplastic skin (2nd row), papilloma (3rd row), squamous cell carcinoma (SCC, 4th row). Bar=250 μm for low and 25 μm for high magnification.
While previous studies have correlated TPA treatment alone with altered Cx43 and Cx26 levels in vivo [109, 110], this clearly is not the direct cause for the increased pS368 staining in hyperplastic skin and papillomas observed here as we noted no increased phosphostaining in adjacent normal skin. However, PKC activity has been shown to be responsible for pS368 levels [52] and may be linked to signaling pathway activation (in hyperplastic skin) as well as oncogenic alterations in papillomas and tumors. Given that pS368 is primarily localized to junctions and increased phosphorylation of this serine causes changes in Cx43 communication [52], its effects on tumor progression would likely be related to channel gating. However, altered assembly or degradation of Cx43 may also be involved. In summary, the level of total Cx43 as well as regulation via phosphorylation of Cx43 on S368 are clearly altered during mouse skin tumorigenesis and indicate that Cx43 could serve a regulatory tumor suppressor function in this tissue type.
6. Concluding Remarks
Many studies performed in tissue culture systems have clearly shown that gap junctional communication can be modified by connexin phosphorylation. Often these studies involved treatment with kinase activators or growth factors that dramatically affect cell biology. Until recently, little was known about whether these phosphorylation events actually occur in vivo. The advent of phosphorylation status specific antibodies has revolutionized the field of cell biology and we believe that it will also open up many new linkages between connexin function and specific biological processes including, development, cell fate determination, growth control and tumor progression. We believe that these regulatory events not only affect gap junctional communication but also affect the proteins that interact with connexins. While Cx43 is phosphorylated at over a dozen sites, only a few commercially available phosphospecific antibodies for Cx43 have been shown to be useful for immunostaining. Even though current available antibodies allow for poor coverage of the total Cx43 phosphorylation events possible, the results that have been obtained are very intriguing. As we gain a better understanding of the consequences of phosphorylation, develop more phosphorylation state specific antibodies, and appreciate the consequences of connexin interactions with other proteins, we believe the list of biological processes affected by connexins in vivo will dramatically grow.
Acknowledgements
Practical considerations have led us to utilize reviews or limit citations of original work, and we apologize for these deficiencies. The work in the authors’ laboratory was supported in part by a grant from the National Institutes Health (GM55632 to PDL).
Footnotes
- Cx
- connexin
- MAPK
- Mitogen-activated protein kinase
- PKC
- protein kinase C
- TPA
- 12-O-tetradeconylphorbol-13-acetate
- NP
- non-phosphorylated
- SDS-PAGE
- sodium dodecylsulfate-polyacrylamide gel electrophoresis.
References
- [1].Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat. Rev. Mol. Cell. Biol. 2003;4:285–94. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- [2].Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- [3].Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol. Rev. 2003;83:1359–400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- [4].Mesnil M. Connexins and cancer. Biol. Cell. 2002;94:493–500. doi: 10.1016/s0248-4900(02)00025-4. [DOI] [PubMed] [Google Scholar]
- [5].White T, Paul D. Genetic diseases and gene knockouts reveal diverse connexin functions. Ann. Rev. Physiology. 1999;61:283–310. doi: 10.1146/annurev.physiol.61.1.283. [DOI] [PubMed] [Google Scholar]
- [6].Wei CJ, Xu X, Lo CW. Connexins and cell signaling in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:811–38. doi: 10.1146/annurev.cellbio.19.111301.144309. [DOI] [PubMed] [Google Scholar]
- [7].Kelsell DP, Dunlop J, Stevens HP, Lench NJ, Laing JN, Parry G, Mueller RF, Leigh IM. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature. 1997;387:80–83. doi: 10.1038/387080a0. [DOI] [PubMed] [Google Scholar]
- [8].Shiels A, Mackay D, Ionides A, Berry V, Moore A, Bhattacharya S. A missense mutation in the human connexin50 gene (GJA8) underlies autosomal dominant “zonular pulverulent” cataract, on chromosome 1q. Am. J. Hum. Genet. 1998;62:526–32. doi: 10.1086/301762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Berry V, Mackay D, Khaliq S, Francis PJ, Hameed A, Anwar K, Mehdi SQ, Newbold RJ, Ionides A, Shiels A, Moore T, Bhattacharya SS. Connexin 50 mutation in a family with congenital “zonular nuclear” pulverulent cataract of Pakistani origin. Hum. Genet. 1999;105:168–70. doi: 10.1007/s004399900094. [DOI] [PubMed] [Google Scholar]
- [10].Bergoffen J, Scherer SS, Wang S, Oronzi M, Scott LJ, Bone DL, Paul K, Chen MW, Lensch PF, Chance KH. Fishbeck, Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science. 1993;262:2039–2042. doi: 10.1126/science.8266101. [DOI] [PubMed] [Google Scholar]
- [11].Spray DC, Dermietzel R. X-linked Charcot-Marie-Tooth disease and other potential gap junction-diseases of the nervous system. Trends Neurosci. 1995;18:256–262. [PubMed] [Google Scholar]
- [12].King TJ, Lampe PD. Mice deficient for the gap junction protein Connexin32 exhibit increased radiation-induced tumorigenesis associated with elevated mitogen-activated protein kinase (p44/Erk1, p42/Erk2) activation. Carcinogenesis. 2004;25:669–80. doi: 10.1093/carcin/bgh071. [DOI] [PubMed] [Google Scholar]
- [13].Moennikes O, Buchmann A, Ott T, Willecke K, Schwarz M. The effect of connexin32 null mutation on hepatocarcinogenesis in different mouse strains. Carcinogenesis. 1999;20:1379–82. doi: 10.1093/carcin/20.7.1379. [DOI] [PubMed] [Google Scholar]
- [14].Temme A, Buchman A, Babriel HC, Nelles E, Scharz M, Willecke K. High incidence of spontaneous and chemically-induced liver tumors in mice deficient for connexin 32. Curr. Biol. 1997;7:713–718. doi: 10.1016/s0960-9822(06)00302-2. [DOI] [PubMed] [Google Scholar]
- [15].King TJ, Lampe PD. The gap junction protein connexin32 is a mouse lung tumor suppressor. Cancer Res. 2004;64:7191–6. doi: 10.1158/0008-5472.CAN-04-0624. [DOI] [PubMed] [Google Scholar]
- [16].Paznekas WA, Boyadjiev SA, Shapiro RE, Daniels O, Wollnik B, Keegan CE, Innis JW, Dinulos MB, Christian C, Hannibal MC, Jabs EW. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am. J. Hum. Genet. 2003;72:408–18. doi: 10.1086/346090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin37. Nature. 1997;385:525–529. doi: 10.1038/385525a0. [DOI] [PubMed] [Google Scholar]
- [18].Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- [19].Eloff BC, Lerner DL, Yamada KA, Schuessler RB, Saffitz JE, Rosenbaum DS. High resolution optical mapping reveals conduction slowing in connexin43 deficient mice. Cardiovasc. Res. 2001;51:681–90. doi: 10.1016/s0008-6363(01)00341-8. [DOI] [PubMed] [Google Scholar]
- [20].Simon AM, Goodenough DA, Paul DL. Mice lacking connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Curr. Biol. 1998;8:295–298. doi: 10.1016/s0960-9822(98)70113-7. [DOI] [PubMed] [Google Scholar]
- [21].White TW, Goodenough DA, Paul DL. Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J. Cell Biol. 1998;143:815–825. doi: 10.1083/jcb.143.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gong X, Li E, Klier G, Huang Q, Wu Y, Lei H, Kumar NM, Horwitz J, Gilula NB. Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell. 1997;91:833–843. doi: 10.1016/s0092-8674(00)80471-7. [DOI] [PubMed] [Google Scholar]
- [23].White TW. Unique and redundant connexin contributions to lens development. Science. 2002;295:319–320. doi: 10.1126/science.1067582. [DOI] [PubMed] [Google Scholar]
- [24].Plum A, Hallas G, Magin T, Dombrowski F, Hagendorff A, Schumacher B, Wolpert C, Kim J, Lamers WH, Evert M, Meda P, Traub O, Willecke K. Unique and shared functions of different connexins in mice. Curr. Biol. 2000;10:1083–1091. doi: 10.1016/s0960-9822(00)00690-4. [DOI] [PubMed] [Google Scholar]
- [25].Maass K, Ghanem A, Kim JS, Saathoff M, Urschel S, Kirfel G, Grummer R, Kretz M, Lewalter T, Tiemann K, Winterhager E, Herzog V, Willecke K. Defective epidermal barrier in neonatal mice lacking the C-terminal region of connexin43. Mol. Biol. Cell. 2004;15:4597–608. doi: 10.1091/mbc.E04-04-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int. J. Biochem. Cell Biol. 2004;36 doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lampe PD, Lau AF. Regulation of gap junctions by phosphorylation of connexins. Arch. Biochem. Biophys. 2000;384:205–215. doi: 10.1006/abbi.2000.2131. [DOI] [PubMed] [Google Scholar]
- [28].Stagg RB, Fletcher WH. The hormone-induced regulation of contact-dependent cell-cell communication by phosphorylation. Endocr. Rev. 1990;11:302–325. doi: 10.1210/edrv-11-2-302. [DOI] [PubMed] [Google Scholar]
- [29].Laird DW. Connexin phosphorylation as a regulatory event linked to gap junction internalization and degradation. Biochim. Biophys. Acta. 2005;1711:172–82. doi: 10.1016/j.bbamem.2004.09.009. [DOI] [PubMed] [Google Scholar]
- [30].Moreno AP. Connexin phosphorylation as a regulatory event linked to channel gating. Biochim. Biophys. Acta. 2005;1711:164–71. doi: 10.1016/j.bbamem.2005.02.016. [DOI] [PubMed] [Google Scholar]
- [31].Solan JL, Lampe PD. Connexin phosphorylation as a regulatory event linked to gap junction channel assembly. Biochim. Biophys. Acta. 2005;1711:154–63. doi: 10.1016/j.bbamem.2004.09.013. [DOI] [PubMed] [Google Scholar]
- [32].Berthoud VM, Westphale EM, Grigoryeva A, Beyer EC. PKC isoenzymes in the chicken lens and TPA-induced effects on intercellular communication. Invest. Ophthalmol. Vis. Sci. 2000;41:850–858. [PubMed] [Google Scholar]
- [33].Mitropoulou G, Bruzzone R. Modulation of perch connexin35 hemi-channels by cyclic AMP requires a protein kinase A phosphorylation site. J. Neurosci. Res. 2003;72:147–57. doi: 10.1002/jnr.10572. [DOI] [PubMed] [Google Scholar]
- [34].Musil LS, Beyer EC, Goodenough DA. Expression of the gap junction protein connexin43 in embryonic chick lens: Molecular cloning, ultrastructural localization, and post-translational phosphorylation. J. Membr. Biol. 1990;116:163–175. doi: 10.1007/BF01868674. [DOI] [PubMed] [Google Scholar]
- [35].Crow DS, Beyer EC, Paul DL, Kobe SS, Lau AF. Phosphorylation of connexin43 gap junction protein in uninfected and Rous sarcoma virus-transformed mammalian fibroblasts. Mol. Cell. Biol. 1990;10:1754–1763. doi: 10.1128/mcb.10.4.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Laird DW, Puranam KL, Revel JP. Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochem. J. 1991;273:67–72. doi: 10.1042/bj2730067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lampe PD. Analyzing phorbol ester effects on gap junction communication: A dramatic inhibition of assembly. J. Cell Biol. 1994;127:1895–1905. doi: 10.1083/jcb.127.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Beardslee M, Laing J, Beyer E, Saffitz J. Rapid turnover of connexin43 in the adult rat heart [see comments] Circ. Res. 1998;83:629–635. doi: 10.1161/01.res.83.6.629. [DOI] [PubMed] [Google Scholar]
- [39].Berthoud VM, Ledbetter MLS, Hertzberg EL, Saez JC. Connexin43 in MDCK cells: Regulation by a tumor-promoting phorbol ester and calcium. Eur. J. Cell Biol. 1992;57:40–50. [PubMed] [Google Scholar]
- [40].Brissette JL, Kumar NM, Gilula NB, Dotto GP. The tumor promoter 12-O-tetradecanoylphorbol-13-acetate and the ras oncogene modulate expression and phosphorylation of gap junction proteins. Mol. Cell. Biol. 1991;11:5364–5371. doi: 10.1128/mcb.11.10.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kadle R, Zhang JT, Nicholson BJ. Tissue-specific distribution of differentially phosphorylated forms of Cx43. Mol. Cell. Biol. 1991;11:363–369. doi: 10.1128/mcb.11.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Musil LS, Cunningham BA, Edelman GM, Goodenough DA. Differential phosphorylation of gap junction protein connexin43 in junctional communication-competent and deficient cell lines. J. Cell Biol. 1990;111:2077–2088. doi: 10.1083/jcb.111.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Musil LS, Goodenough DA. Biochemical analysis of connexin43 intracellular transport, phosphorylation and assembly into gap junctional plaques. J. Cell Biol. 1991;115:1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Solan JL, Fry MD, TenBroek EM, Lampe PD. Connexin43 phosphorylation at S368 is acute during S and G2/M and in response to protein kinase C activation. J. Cell Sci. 2003;116:2203–2211. doi: 10.1242/jcs.00428. [DOI] [PubMed] [Google Scholar]
- [45].Lampe PD, Kurata WE, Warn-Cramer B, Lau AF. Formation of a distinct connexin43 phosphoisoform in mitotic cells is dependent upon p34cdc2 kinase. J. Cell Sci. 1998;111:833–841. doi: 10.1242/jcs.111.6.833. [DOI] [PubMed] [Google Scholar]
- [46].Laird DL, Castillo M, Kasprzak L. Gap junction turnover, intracellular trafficking, and phosphorylation of connexin43 in Brefeldin A-treated rat mammary tumor cells. J. Cell Biol. 1995;131:1193–1203. doi: 10.1083/jcb.131.5.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Warn-Cramer BJ, Lampe PD, Kurata WE, Kanemitsu MY, Loo LWM, Eckhart W, Lau AF. Characterization of the MAP kinase phosphorylation sites on the connexin43 gap junction protein. J. Biol. Chem. 1996;271:3779–3786. doi: 10.1074/jbc.271.7.3779. [DOI] [PubMed] [Google Scholar]
- [48].Swenson KI, Piwnica-Worms H, McNamee H, Paul DL. Tyrosine phosphorylation of the gap junction protein connexin43 is required for pp60src-induced inhibition of communication. Cell Regul. 1990;1:989–1002. doi: 10.1091/mbc.1.13.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Berthoud VM, Beyer EC, Kurata WE, Lau AF, Lampe PD. The gap junction protein connexin 56 is phosphorylated in the intracellular loop and the carboxy-terminal region. Eur. J. Biochem. 1997;244:89–97. doi: 10.1111/j.1432-1033.1997.00089.x. [DOI] [PubMed] [Google Scholar]
- [50].Reynhout JK, Lampe PD, Johnson RG. An activator of protein kinase C inhibits gap junction communication between cultured bovine lens cells. Exp. Cell Res. 1992;198:337–342. doi: 10.1016/0014-4827(92)90388-o. [DOI] [PubMed] [Google Scholar]
- [51].Martinez AD, Hayrapetyan V, Moreno AP, Beyer EC. Connexin43 and connexin45 form heteromeric gap junction channels in which individual components determine permeability and regulation. Circ. Res. 2002;90:1100–7. doi: 10.1161/01.res.0000019580.64013.31. [DOI] [PubMed] [Google Scholar]
- [52].Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J. Cell Biol. 2000;126:1503–1512. doi: 10.1083/jcb.149.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Saez JC, Nairn AC, Czernik AJ, Fishman GI, Spray DC, Hertzberg EL. Phosphorylation of connexin43 and the regulation of neonatal rat cardiac myocyte gap junctions. J. Mol. Cell. Cardiol. 1997;29:2131–2145. doi: 10.1006/jmcc.1997.0447. [DOI] [PubMed] [Google Scholar]
- [54].Shah MM, Martinez A-M, Fletcher WH. The connexin43 gap junction protein is phosphorylated by protein kinase A and protein kinase C: In vivo and in vtitro studies. Mol. Cell. Biochem. 2002;238:57–68. doi: 10.1023/a:1019902920693. [DOI] [PubMed] [Google Scholar]
- [55].Doble BW, Dang X, Ping P, Fandrich RR, Nickel BE, Jin Y, Cattini PA, Kardami E. Phosphorylation of serine 262 in the gap junction protein connexin-43 regulates DNA synthesis in cell-cell contact forming cardiomyocytes. J. Cell Sci. 2004;117:507–14. doi: 10.1242/jcs.00889. [DOI] [PubMed] [Google Scholar]
- [56].Bowling N, Huang X, Sandusky GE, Fouts RL, Mintze K, Esterman M, Allen PD, Maddi R, McCall E, Vlahos CJ. Protein kinase C-alpha and -epsilon modulate connexin-43 phosphorylation in human heart. J. Mol. Cell. Cardiol. 2001;33:789–98. doi: 10.1006/jmcc.2000.1349. [DOI] [PubMed] [Google Scholar]
- [57].Doble BW, Ping P, Kardami E. The epsilon subtype of protein kinase C is required for cardiomyocyte connexin-43 phosphorylation. Circ. Res. 2000;86:293–301. doi: 10.1161/01.res.86.3.293. [DOI] [PubMed] [Google Scholar]
- [58].Cooper CD, Lampe PD. Casein kinase 1 regulates connexin43 gap junction assembly. J. Biol. Chem. 2002;277:44962–44968. doi: 10.1074/jbc.M209427200. [DOI] [PubMed] [Google Scholar]
- [59].Yogo K, Ogawa T, Akiyama M, Ishida N, Takeya T. Identification and functional analysis of novel phosphorylation sites in Cx43 in rat primary granulosa cells. FEBS Lett. 2002;531:132–136. doi: 10.1016/s0014-5793(02)03441-5. [DOI] [PubMed] [Google Scholar]
- [60].Lin R, Warn-Cramer BJ, Kurata WE, Lau AF. v-Src phosphorylation of connexin 43 on Tyr247 and Tyr265 disrupts gap junctional communication. J. Cell Biol. 2001;154:815–27. doi: 10.1083/jcb.200102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kanemitsu MY, Jiang W, Eckhart W. Cdc2-mediated phosphorylation of the gap junction protein, connexin43, during mitosis. Cell Growth Differ. 1998;9:13–21. [PubMed] [Google Scholar]
- [62].Nagy JI, Li WEI, Roy C, Doble BW, Gilchrist JS, Kardami E, Hertzberg EL. Selective monoclonal antibody recognition and cellular localization of an unphosporylated form of connexin43. Exper. Cell Res. 1997;236:127–136. doi: 10.1006/excr.1997.3716. [DOI] [PubMed] [Google Scholar]
- [63].Cruciani V, Mikalsen SO. Stimulated phosphorylation of intracellular connexin43. Exp. Cell Res. 1999;251:285–298. doi: 10.1006/excr.1999.4574. [DOI] [PubMed] [Google Scholar]
- [64].Leykauf K, Durst M, Alonso A. Phosphorylation and subcellular distribution of connexin43 in normal and stressed cells. Cell Tissue Res. 2003;311:23–30. doi: 10.1007/s00441-002-0645-5. [DOI] [PubMed] [Google Scholar]
- [65].Severs NJ, Coppen SR, Dupont E, Yeh HI, Ko YS, Matsushita T. Gap junction alterations in human cardiac disease. Cardiovasc. Res. 2004;62:368–77. doi: 10.1016/j.cardiores.2003.12.007. [DOI] [PubMed] [Google Scholar]
- [66].Britz-Cunningham SH, Shah MM, Zuppan CW, Fletcher WH. Mutations of the connexin43 gap junction gene in patients with heart malformations and defects of laterality. New Engl. J. Med. 1995;332:1323–1329. doi: 10.1056/NEJM199505183322002. [DOI] [PubMed] [Google Scholar]
- [67].Splitt MP, Tsai MY, Burn J, Goodship JA. Absence of mutations in the regulatory domain of the gap junction protein connexin43 in patients with visceroatrial heterotaxy. Heart. 1997;77:369–370. doi: 10.1136/hrt.77.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Debrus S, Tuffery S, Matsuoka R, Galal O, Sarda P, Sauer U, Bozio A, Tanman B, Toutain A, Claustres M, Le Paslier D, Bouvagnet P. Lack of evidence for connexin 43 gene mutations in human autosomal recessive lateralization defects. J. Mol. Cell. Cardiol. 1997;29:1423–1431. doi: 10.1006/jmcc.1997.0380. [DOI] [PubMed] [Google Scholar]
- [69].Dasgupta C, Martinez AM, Zuppan CW, Shah MM, Bailey LL, Fletcher WH. Identification of connexin43 (alpha1) gap junction gene mutations in patients with hypoplastic left heart syndrome by denaturing gradient gel electrophoresis (DGGE) Mutat. Res. 2001;479:173–86. doi: 10.1016/s0027-5107(01)00160-9. [DOI] [PubMed] [Google Scholar]
- [70].Kirchhoff S, Nelles E, Hagendorff A, Kruger O, Traub O, Willecke K. Reduced cardiac conduction velocity and predisposition to arrhythmias in connexin40-deficient mice. Curr. Biol. 1998;8:299–302. doi: 10.1016/s0960-9822(98)70114-9. [DOI] [PubMed] [Google Scholar]
- [71].Hagendorff A, Schumacher B, Kirchhoff S, Luderitz B, Willecke K. Conduction disturbances and increased atrial vulnerability in Connexin40-deficient mice analyzed by transesophageal stimulation. Circulation. 1999;99:1508–15. doi: 10.1161/01.cir.99.11.1508. [DOI] [PubMed] [Google Scholar]
- [72].Bevilacqua LM, Simon AM, Maguire CT, Gehrmann J, Wakimoto H, Paul DL, Berul CI. A targeted disruption in connexin40 leads to distinct atrioventricular conduction defects. J. Interv. Card. Electrophysiol. 2000;4:459–67. doi: 10.1023/a:1009800328836. [DOI] [PubMed] [Google Scholar]
- [73].Vaidya D, Tamaddon HS, Lo CW, Taffet SM, Delmar M, Morley GE, Jalife J. Null mutation of connexin43 causes slow propagation of ventricular activation in the late stages of mouse embryonic development. Circ. Res. 2001;88:1196–202. doi: 10.1161/hh1101.091107. [DOI] [PubMed] [Google Scholar]
- [74].Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H, Fishman GI. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ. Res. 2001;88:333–9. doi: 10.1161/01.res.88.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Theis M, Mas C, Doring B, Kruger O, Herrera P, Meda P, Willecke K. General and conditional replacement of connexin43-coding DNA by a lacZ reporter gene for cell-autonomous analysis of expression. Cell Commun. Adhes. 2001;8:383–6. doi: 10.3109/15419060109080758. [DOI] [PubMed] [Google Scholar]
- [76].Jain SK, Schuessler RB, Saffitz JE. Mechanisms of delayed electrical uncoupling induced by ischemic preconditioning. Circ. Res. 2003;92:1138–44. doi: 10.1161/01.RES.0000074883.66422.C5. [DOI] [PubMed] [Google Scholar]
- [77].Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, Yamada KA, Kleber AG, Schuessler RB, Saffitz JE. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ. Res. 2000;87:656–62. doi: 10.1161/01.res.87.8.656. [DOI] [PubMed] [Google Scholar]
- [78].Force T, Pombo CM, Avruch JA, Bonventre JV, Kyriakis JM. Stress-activated protein kinases in cardiovascular disease. Circ. Res. 1996;78:947–53. doi: 10.1161/01.res.78.6.947. [DOI] [PubMed] [Google Scholar]
- [79].Schulz R, Gres P, Skyschally A, Duschin A, Belosjorow S, Konietzka I, Heusch G. Ischemic preconditioning preserves connexin 43 phosphorylation during sustained ischemia in pig hearts in vivo. FASEB J. 2003;17:1355–7. doi: 10.1096/fj.02-0975fje. [DOI] [PubMed] [Google Scholar]
- [80].Duncan JC, Fletcher WH. alpha 1 Connexin (connexin43) gap junctions and activities of cAMP-dependent protein kinase and protein kinase C in developing mouse heart. Dev. Dyn. 2002;223:96–107. doi: 10.1002/dvdy.1232. [DOI] [PubMed] [Google Scholar]
- [81].Yin X, Jedrzejewski PT, Jiang JX. Casein kinase II phosphorylates lens connexin 45.6 and is involved in its degradation. J. Biol. Chem. 2000;275:6850–6856. doi: 10.1074/jbc.275.10.6850. [DOI] [PubMed] [Google Scholar]
- [82].Richard G. Connexins: a connection with the skin. Exp. Dermatol. 2000;9:77–96. doi: 10.1034/j.1600-0625.2000.009002077.x. [DOI] [PubMed] [Google Scholar]
- [83].van Steensel MA. Gap junction diseases of the skin. Am. J. Med. Genet. C Semin Med. Genet.C Semin Med. Genet. 2004;131C:12–9. doi: 10.1002/ajmg.c.30030. [DOI] [PubMed] [Google Scholar]
- [84].Sawey MJ, Goldschmidt MH, Risek B, Gilula NB, Lo CW. Perturbation in connexin 43 and connexin 26 gap-junction expression in mouse skin hyperplasia and neoplasia. Mol. Carcinog. 1996;17:49–61. doi: 10.1002/(SICI)1098-2744(199610)17:2<49::AID-MC1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- [85].Kamibayashi Y, Oyamada Y, Mori M, Oyamada M. Aberrant expression of gap junction proteins (connexins) is associated with tumor progression during multistage mouse skin carcinogenesis in vivo. Carcinogenesis. 1995;16:1287–97. doi: 10.1093/carcin/16.6.1287. [DOI] [PubMed] [Google Scholar]
- [86].Budunova IV, Carbajal S, Slaga TJ. The expression of gap junctional proteins during different stages of mouse skin carcinogenesis. Carcinogenesis. 1995;16:2717–24. doi: 10.1093/carcin/16.11.2717. [DOI] [PubMed] [Google Scholar]
- [87].Arita K, Akiyama M, Tsuji Y, McMillan JR, Eady RA, Shimizu H. Changes in gap junction distribution and connexin expression pattern during human fetal skin development. J. Histochem. Cytochem. 2002;50:1493–500. doi: 10.1177/002215540205001109. [DOI] [PubMed] [Google Scholar]
- [88].Fitzgerald DJ, Fusenig NE, Boukamp P, Piccoli C, Mesnil M, Yamasaki H. Expression and function of connexin in normal and transformed human keratinocytes in culture. Carcinogenesis. 1994;15:1859–1865. doi: 10.1093/carcin/15.9.1859. [DOI] [PubMed] [Google Scholar]
- [89].Di WL, Rugg EL, Leigh IM, Kelsell DP. Multiple epidermal connexins are expressed in different keratinocyte subpopulations including connexin 31. J. Invest. Dermatol. 2001;117:958–64. doi: 10.1046/j.0022-202x.2001.01468.x. [DOI] [PubMed] [Google Scholar]
- [90].Salomon D, Masgrau E, Vischer S, Ullrich S, Dupont E, Sappino P, Saurat J-H, Meda P. Topography of mammalian connexins in human skin. J. Invest. Dermatol. 1994;103:240–247. doi: 10.1111/1523-1747.ep12393218. [DOI] [PubMed] [Google Scholar]
- [91].Risek B, Klier FG, Gilula NB. Multiple gap junction genes are utilized during rat skin and hair development. Development. 1992;116:639–651. doi: 10.1242/dev.116.3.639. [DOI] [PubMed] [Google Scholar]
- [92].Goliger JA, Paul DL. Expression of gap junction proteins Cx26, Cx31.1, Cx37, and Cx43 in developing and mature rat epidermis. Develop. Dynam. 1994;200:1–13. doi: 10.1002/aja.1002000102. [DOI] [PubMed] [Google Scholar]
- [93].Lampe PD, Nguyen BP, Gil S, Usui M, Olerud J, Takada Y, Carter WG. Cellular interaction of integrin α3β1 with laminin 5 promotes gap junctional communication. J. Cell Biol. 1998;143:1735–1747. doi: 10.1083/jcb.143.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kretz M, Euwens C, Hombach S, Eckardt D, Teubner B, Traub O, Willecke K, Ott T. Altered connexin expression and wound healing in the epidermis of connexin-deficient mice. J. Cell Sci. 2003;116:3443–52. doi: 10.1242/jcs.00638. [DOI] [PubMed] [Google Scholar]
- [95].Clark RAF. Cutaneous tissue repair: basic biological considerations. J. Am. Acad. Derm. 1985;13:701–725. doi: 10.1016/s0190-9622(85)70213-7. [DOI] [PubMed] [Google Scholar]
- [96].Grinnell F. Wound repair, keratinocyte activation and integrin modulation. J. Cell Sci. 1992;101:1–5. doi: 10.1242/jcs.101.1.1. [DOI] [PubMed] [Google Scholar]
- [97].Gailit J, Clark RA. Wound repair in the context of extracellular matrix. Curr. Opin. Cell Biol. 1994;6:717–25. doi: 10.1016/0955-0674(94)90099-x. [DOI] [PubMed] [Google Scholar]
- [98].Goliger JA, Paul DL. Wounding alters epidermal connexin expression and gap junction-mediated intercellular communication. Mol. Biol. Cell. 1995;6:1491–1501. doi: 10.1091/mbc.6.11.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- [100].Saitoh M, Oyamada M, Oyamada Y, Kaku T, Mori M. Changes in the expression of gap junction proteins (connexins) in hamster tongue epithelium during would healing and carcinogenesis. Carcinogenesis. 1997;18:1319–1328. doi: 10.1093/carcin/18.7.1319. [DOI] [PubMed] [Google Scholar]
- [101].Qiu C, Coutinho P, Frank S, Franke S, Law LY, Martin P, Green CR, Becker DL. Targeting connexin43 expression accelerates the rate of wound repair. Curr. Biol. 2003;13:1697–703. doi: 10.1016/j.cub.2003.09.007. [DOI] [PubMed] [Google Scholar]
- [102].Temme A, Ott T, Dombrowski F, Willecke K. The extent of synchronous initiation and termination of DNA synthesis in regenerating mouse liver is dependent on connexin32 expressing gap junctions. J. Hepatol. 2000;32:627–35. doi: 10.1016/s0168-8278(00)80225-1. [DOI] [PubMed] [Google Scholar]
- [103].Richards TS, Dunn CA, Carter WG, Usui ML, Olerud JE, Lampe PD. Protein kinase C spatially and temporally regulates gap junctional communication during human wound repair via phosphorylation of connexin43 on serine368. J. Cell Biol. 2004;167:555–62. doi: 10.1083/jcb.200404142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Holden PR, McGuire B, Stoler A, Balmain A, Pitts JD. Changes in gap junctional intercellular communication in mouse skin carcinogenesis. Carcinogenesis. 1997;18:15–21. doi: 10.1093/carcin/18.1.15. [DOI] [PubMed] [Google Scholar]
- [105].Yamakage K, Omori Y, Zaidan-Dagli ML, Cros MP, Yamasaki H. Induction of skin papillomas, carcinomas, and sarcomas in mice in which the connexin 43 gene is heterologously deleted. J. Invest. Dermatol. 2000;114:289–94. doi: 10.1046/j.1523-1747.2000.00873.x. [DOI] [PubMed] [Google Scholar]
- [106].Tada J, Hashimoto K. Ultrastructural localization of gap junction protein connexin 43 in normal human skin, basal cell carcinoma, and squamous cell carcinoma. J. Cutan. Pathol. 1997;24:628–35. doi: 10.1111/j.1600-0560.1997.tb01094.x. [DOI] [PubMed] [Google Scholar]
- [107].Gibson DF, Bikle DD, Harris J, Goldberg GS. The expression of the gap junctional protein Cx43 is restricted to proliferating and non differentiated normal and transformed keratinocytes. Exp. Dermatol. 1997;6:167–74. doi: 10.1111/j.1600-0625.1997.tb00201.x. [DOI] [PubMed] [Google Scholar]
- [108].Brissette JL, Kumar NM, Gilula NB, Hall JE, Dotto GP. Switch in gap junction protein expression is associated with selective changes in junctional permeability during keratinocyte differentiation. Proc. Natl. Acad. Sci. U S A. 1994;91:6453–7. doi: 10.1073/pnas.91.14.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Budunova IV, Carbajal S, Slaga TJ. Effect of diverse tumor promoters on the expression of gap-junctional proteins connexin (Cx)26, Cx31.1, and Cx43 in SENCAR mouse epidermis. Mol. Carcinog. 1996;15:202–14. doi: 10.1002/(SICI)1098-2744(199603)15:3<202::AID-MC6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- [110].Risek B, Pozzi A, Gilula NB. Modulation of gap junction expression during transient hyperplasia of rat epidermis. J. Cell Sci. 1998;111:1395–404. doi: 10.1242/jcs.111.10.1395. [DOI] [PubMed] [Google Scholar]