Abstract
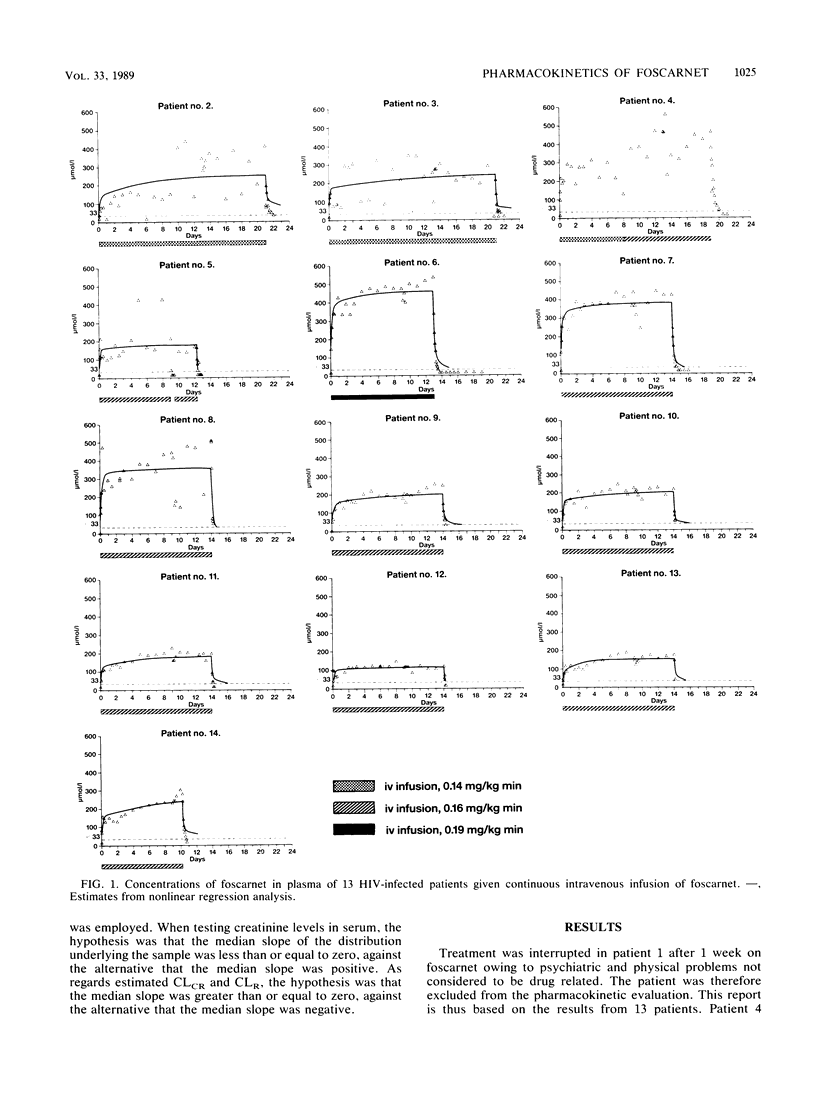
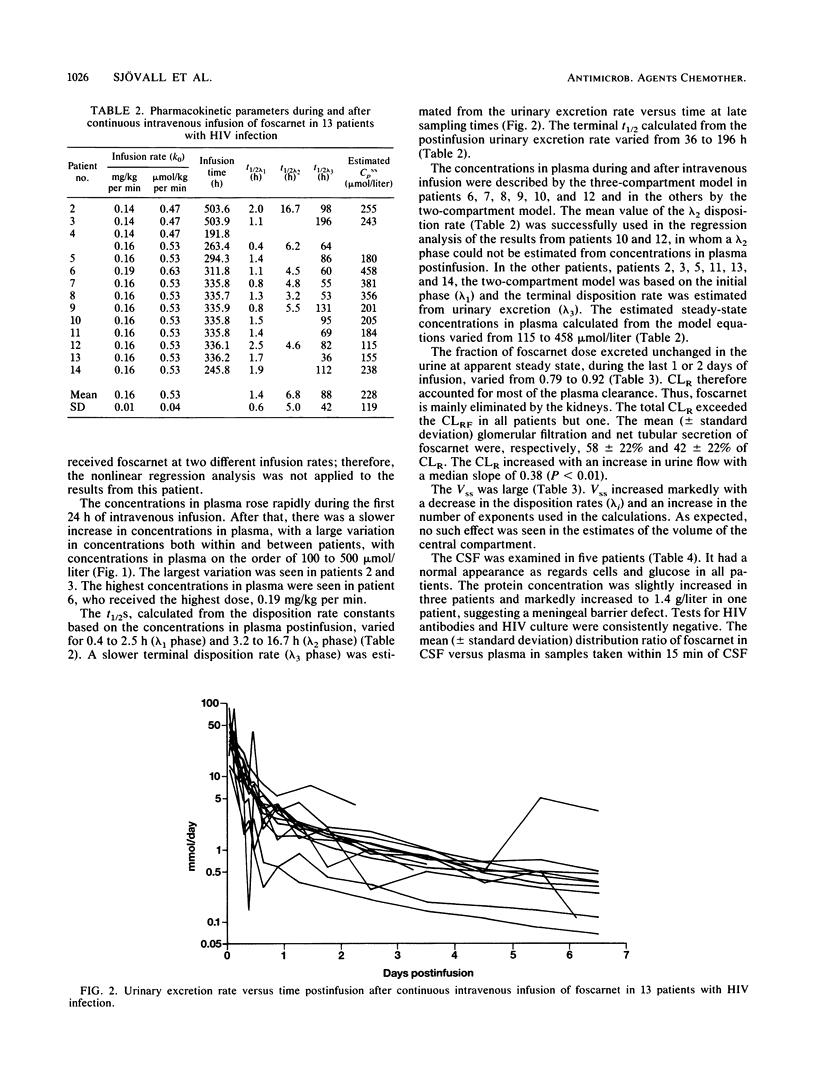
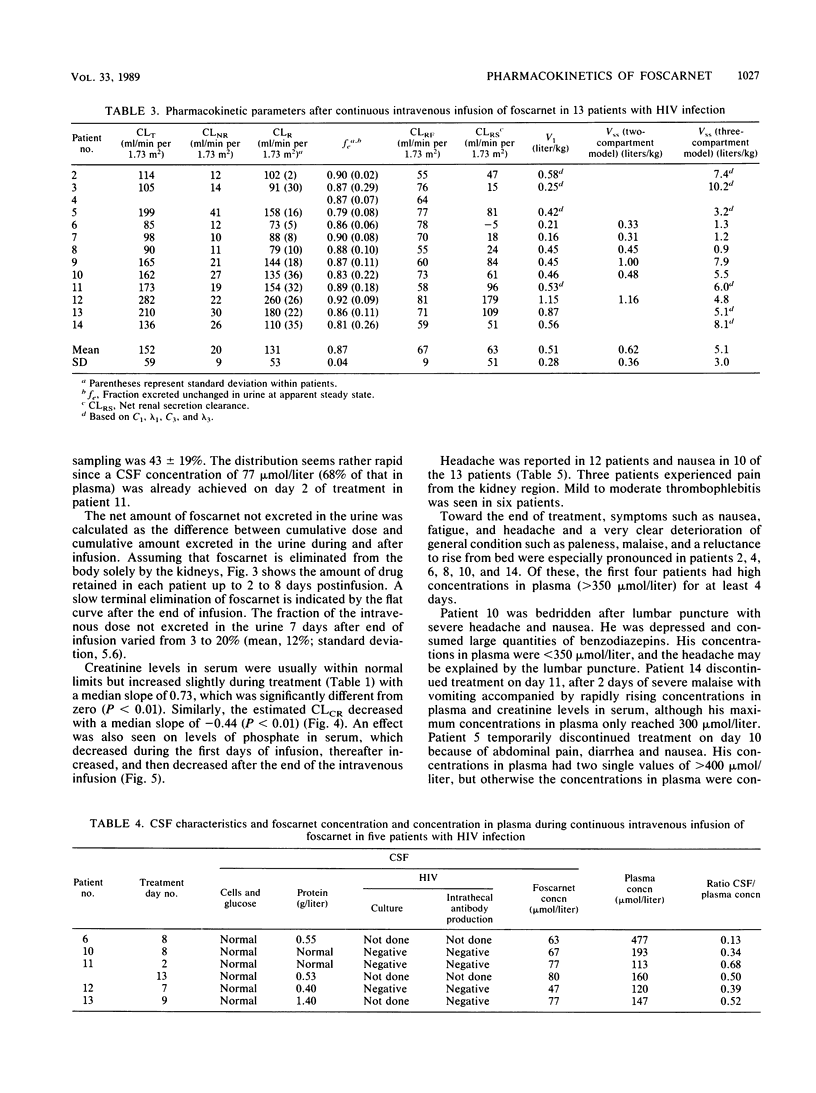
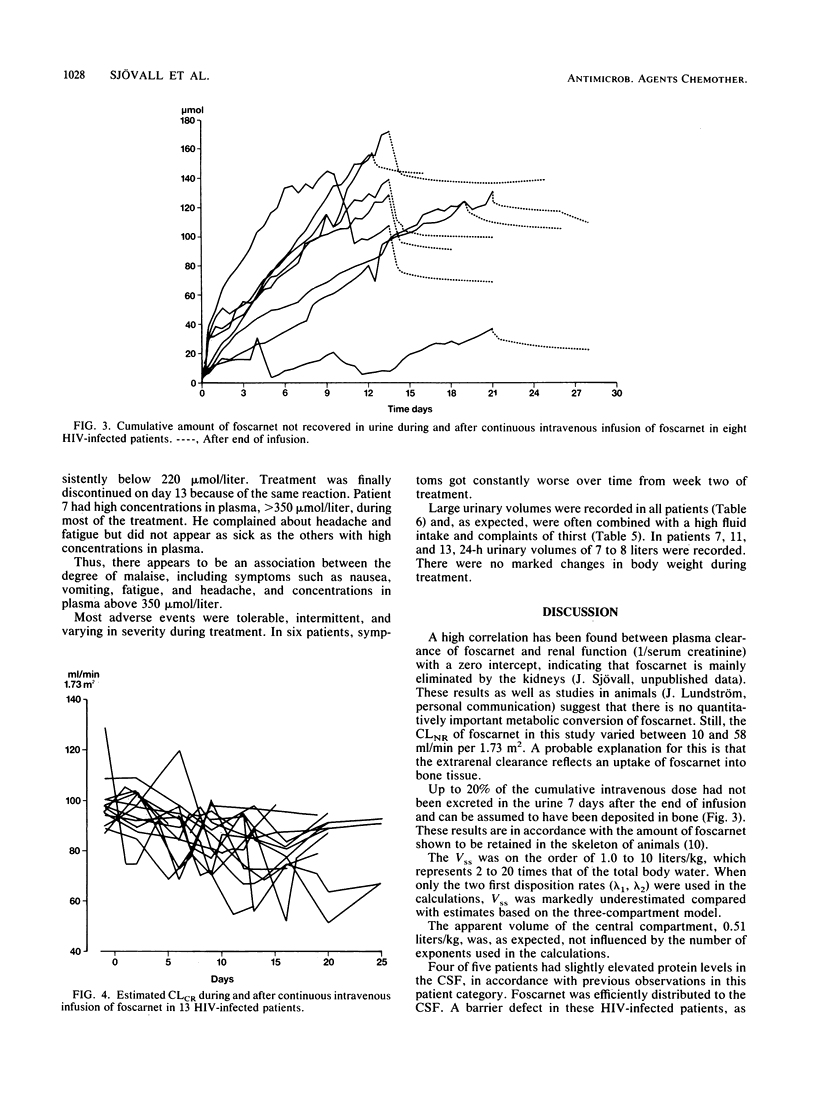
To investigate the pharmacokinetics and effects of intravenous foscarnet, 13 relatively healthy male patients with human immunodeficiency virus infection and a mean CD4+ lymphocyte value of 0.45 x 10(-9) cells per liter were given a continuous intravenous infusion of foscarnet (0.14 to 0.19 mg/kg per min) for 8 to 21 days. Blood and urine samples were taken during and after drug administration to monitor foscarnet concentrations. Lumbar puncture was performed during the infusion in five patients. The concentrations in plasma showed large variations both within and between patients. The disposition of foscarnet could be explained by a triexponential equation (t1/2 lambda 1, 0.40 to 2.52 h; t1/2 lambda 2, 3.20 to 16.7 h; t1/2 lambda 3, 36 to 196 h). Renal clearance accounted for most of the plasma clearance, the difference probably reflecting the passage of foscarnet into bone. Up to 20% of the cumulative dose may have been deposited in bone 7 days postinfusion. Foscarnet was distributed to the cerebrospinal fluid in a concentration varying from 13 to 68% of the simultaneous concentration in plasma. Polyuria and polydipsia were recorded in all patients. There appears to be an association between the degree of malaise, including symptoms such as nausea, vomiting, fatigue, and headache, and concentrations in plasma above 350 mumol/liter.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergdahl S., Sönnerborg A., Larsson A., Strannegard O. Declining levels of HIV P24 antigen in serum during treatment with foscarnet. Lancet. 1988 May 7;1(8593):1052–1052. doi: 10.1016/s0140-6736(88)91867-3. [DOI] [PubMed] [Google Scholar]
- Cockcroft D. W., Gault M. H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Farthing C. F., Dalgleish A. G., Clark A., McClure M., Chanas A., Gazzard B. G. Phosphonoformate (foscarnet): a pilot study in AIDS and AIDS related complex. AIDS. 1987 May;1(1):21–25. [PubMed] [Google Scholar]
- Gaub J., Pedersen C., Poulsen A. G., Mathiesen L. R., Ulrich K., Lindhardt B. O., Faber V., Gerstoft J., Hofmann B., Lernestedt J. O. The effect of foscarnet (phosphonoformate) on human immunodeficiency virus isolation, T-cell subsets and lymphocyte function in AIDS patients. AIDS. 1987 May;1(1):27–33. [PubMed] [Google Scholar]
- Jacobson M. A., Crowe S., Levy J., Aweeka F., Gambertoglio J., McManus N., Mills J. Effect of Foscarnet therapy on infection with human immunodeficiency virus in patients with AIDS. J Infect Dis. 1988 Oct;158(4):862–865. [PubMed] [Google Scholar]
- Klintmalm G., Lönnqvist B., Oberg B., Gahrton G., Lernestedt J. O., Lundgren G., Ringdén O., Robert K. H., Wahren B., Groth C. G. Intravenous foscarnet for the treatment of severe cytomegalovirus infection in allograft recipients. Scand J Infect Dis. 1985;17(2):157–163. doi: 10.3109/inf.1985.17.issue-2.06. [DOI] [PubMed] [Google Scholar]
- Oberg B. Antiviral effects of phosphonoformate (PFA, foscarnet sodium). Pharmacol Ther. 1982;19(3):387–415. doi: 10.1016/0163-7258(82)90074-2. [DOI] [PubMed] [Google Scholar]
- Pettersson K. J., Nordgren T., Westerlund D. Determination of phosphonoformate (foscarnet) in biological fluids by ion-pair reversed-phase liquid chromatography. J Chromatogr. 1989 Mar 24;488(2):447–455. doi: 10.1016/s0378-4347(00)82968-0. [DOI] [PubMed] [Google Scholar]
- Ringdén O., Lönnqvist B., Paulin T., Ahlmén J., Klintmalm G., Wahren B., Lernestedt J. O. Pharmacokinetics, safety and preliminary clinical experiences using foscarnet in the treatment of cytomegalovirus infections in bone marrow and renal transplant recipients. J Antimicrob Chemother. 1986 Mar;17(3):373–387. doi: 10.1093/jac/17.3.373. [DOI] [PubMed] [Google Scholar]
- Sandstrom E. G., Kaplan J. C., Byington R. E., Hirsch M. S. Inhibition of human T-cell lymphotropic virus type III in vitro by phosphonoformate. Lancet. 1985 Jun 29;1(8444):1480–1482. doi: 10.1016/s0140-6736(85)92255-x. [DOI] [PubMed] [Google Scholar]
- Sarin P. S., Taguchi Y., Sun D., Thornton A., Gallo R. C., Oberg B. Inhibition of HTLV-III/LAV replication by foscarnet. Biochem Pharmacol. 1985 Nov 15;34(22):4075–4079. doi: 10.1016/0006-2952(85)90392-2. [DOI] [PubMed] [Google Scholar]
- Sjöström P. A., Odlind B. G., Wolgast M. Extensive tubular secretion and reabsorption of creatinine in humans. Scand J Urol Nephrol. 1988;22(2):129–131. doi: 10.1080/00365599.1988.11690398. [DOI] [PubMed] [Google Scholar]
- Sjövall J., Karlsson A., Ogenstad S., Sandström E., Saarimäki M. Pharmacokinetics and absorption of foscarnet after intravenous and oral administration to patients with human immunodeficiency virus. Clin Pharmacol Ther. 1988 Jul;44(1):65–73. doi: 10.1038/clpt.1988.114. [DOI] [PubMed] [Google Scholar]
- Szczepanska-Konkel M., Yusufi A. N., VanScoy M., Webster S. K., Dousa T. P. Phosphonocarboxylic acids as specific inhibitors of Na+-dependent transport of phosphate across renal brush border membrane. J Biol Chem. 1986 May 15;261(14):6375–6383. [PubMed] [Google Scholar]
- Tucker G. T. Measurement of the renal clearance of drugs. Br J Clin Pharmacol. 1981 Dec;12(6):761–770. doi: 10.1111/j.1365-2125.1981.tb01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley S. L., Chew E., Read S. E., Vellend H., Salit I., Rachlis A., Fanning M. M. Treatment of cytomegalovirus retinitis with trisodium phosphonoformate hexahydrate (Foscarnet). J Infect Dis. 1988 Mar;157(3):569–572. doi: 10.1093/infdis/157.3.569. [DOI] [PubMed] [Google Scholar]
- Yusufi A. N., Szczepanska-Konkel M., Kempson S. A., McAteer J. A., Dousa T. P. Inhibition of human renal epithelial Na+/Pi cotransport by phosphonoformic acid. Biochem Biophys Res Commun. 1986 Sep 14;139(2):679–686. doi: 10.1016/s0006-291x(86)80044-4. [DOI] [PubMed] [Google Scholar]


