Abstract
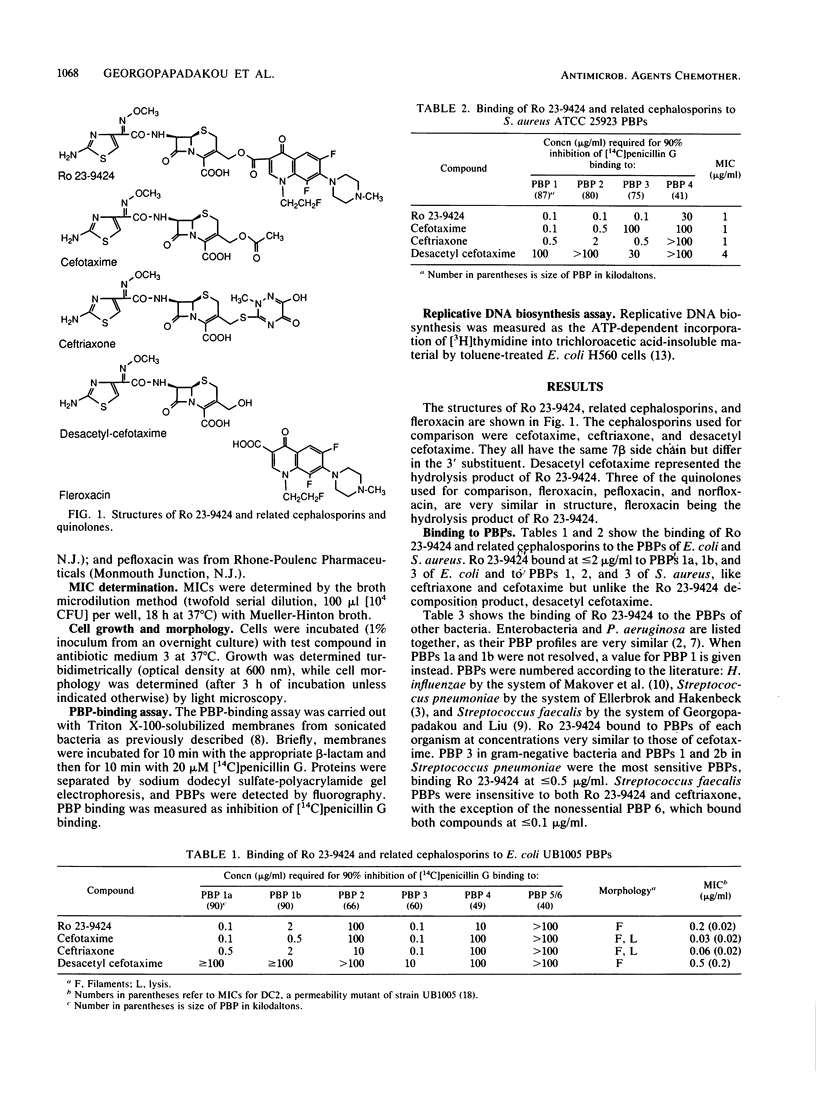
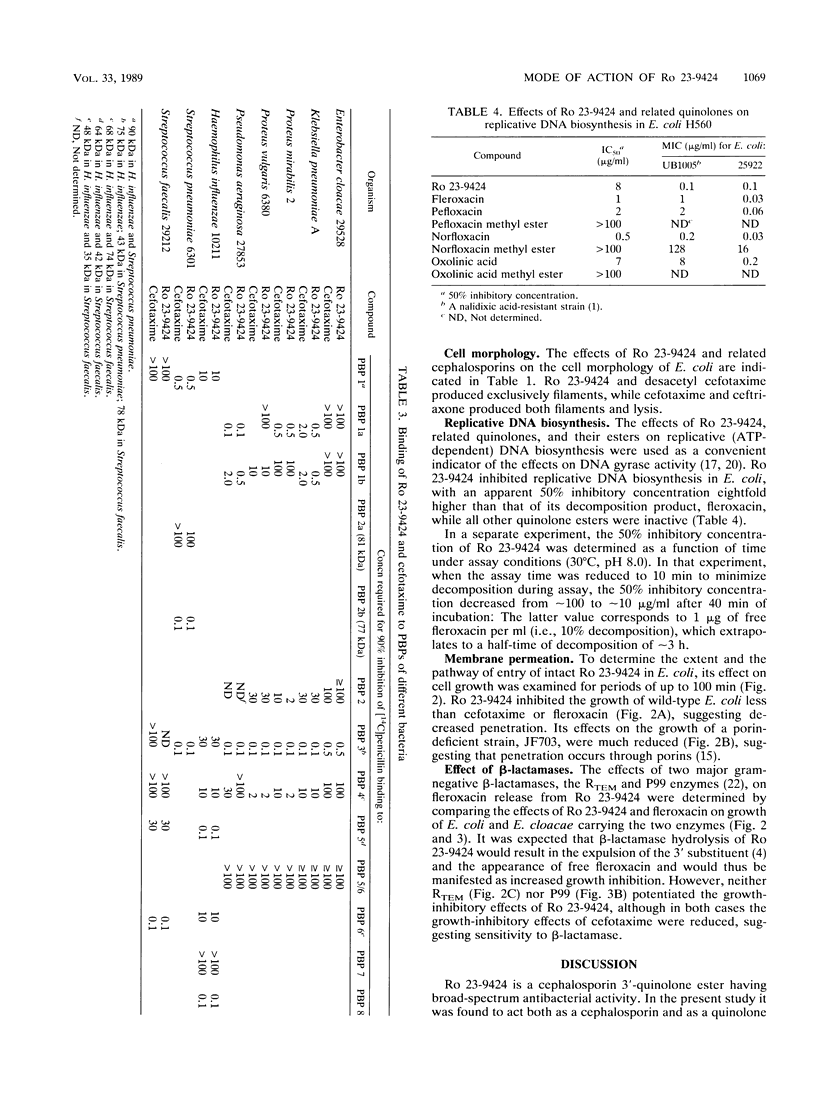
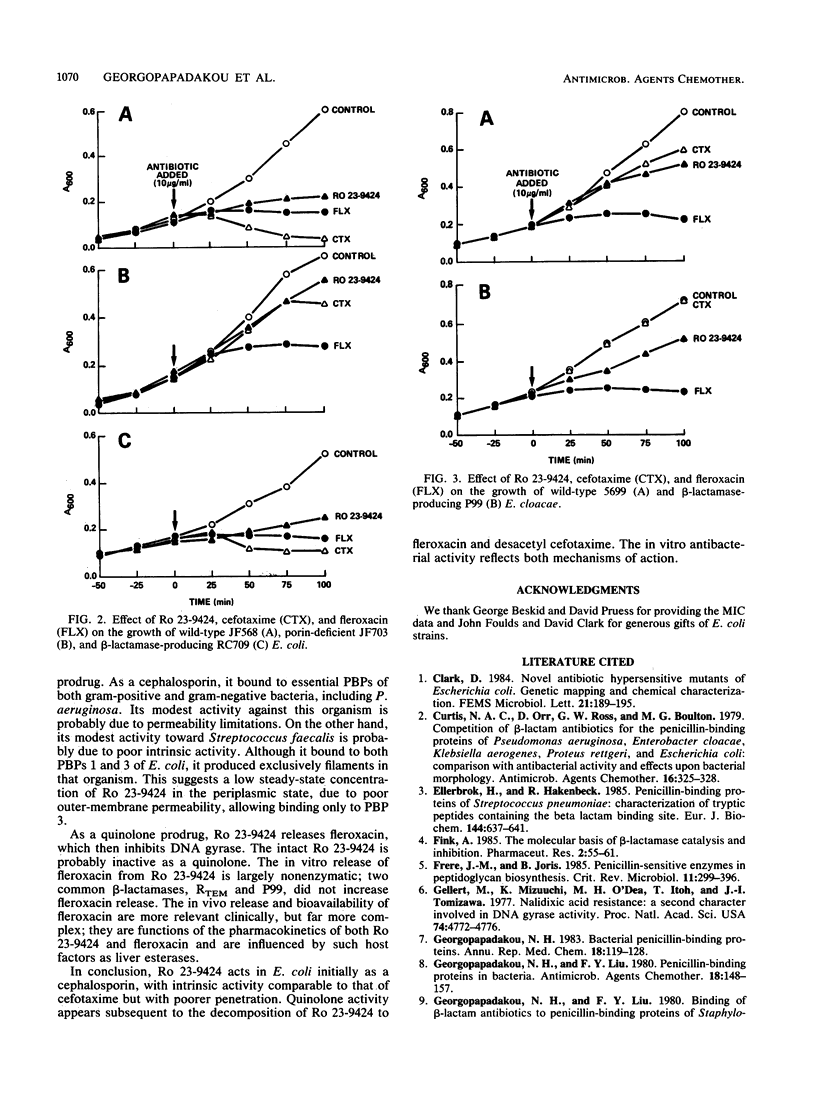
Ro 23-9424 is a broad-spectrum antibacterial agent composed of a cephalosporin and a quinolone moiety. Its biological properties were compared with those of its two components and structurally related cephalosporins and quinolones. Like ceftriaxone and cefotaxime but unlike its decomposition product, desacetyl cefotaxime, Ro 23-9424 bound at less than or equal to 2 micrograms/ml to the essential penicillin-binding proteins 1b and 3 of Escherichia coli and 1, 2, and 3 of Staphylococcus aureus. In E. coli, Ro 23-9424 produced filaments exclusively and decreased cell growth; cefotaxime produced both filaments and lysis. Like its decomposition product fleroxacin but unlike quinolone esters, Ro 23-9424 also inhibited replicative DNA biosynthesis in E. coli. In an E. coli strain lacking OmpF, growth continued after addition of Ro 23-9424, decreased after addition of cefotaxime, and stopped immediately after addition of fleroxacin. The results, together with the chemical stability of Ro 23-9424 (half-life, approximately 3 h at pH 7.4 and 37 degrees C), suggest that in E. coli the compound acts initially as a cephalosporin with intrinsic activity comparable to that of cefotaxime but with poorer penetration. Subsequent to the decomposition of Ro 23-9424 to fleroxacin and desacetyl cefotaxime, quinolone activity appears. The in vitro antibacterial activity reflects both mechanisms of action.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Curtis N. A., Orr D., Ross G. W., Boulton M. G. Competition of beta-lactam antibiotics for the penicillin-binding proteins of Pseudomonas aeruginosa, Enterobacter cloacae, Klebsiella aerogenes, Proteus rettgeri, and Escherichia coli: comparison with antibacterial activity and effects upon bacterial morphology. Antimicrob Agents Chemother. 1979 Sep;16(3):325–328. doi: 10.1128/aac.16.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerbrok H., Hakenbeck R. Penicillin-binding proteins of Streptococcus pneumoniae: characterization of tryptic peptides containing the beta-lactam-binding site. Eur J Biochem. 1984 Nov 2;144(3):637–641. doi: 10.1111/j.1432-1033.1984.tb08512.x. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Joris B. Penicillin-sensitive enzymes in peptidoglycan biosynthesis. Crit Rev Microbiol. 1985;11(4):299–396. doi: 10.3109/10408418409105906. [DOI] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Itoh T., Tomizawa J. I. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Liu F. Y. Binding of beta-lactam antibiotics to penicillin-binding proteins of Staphylococcus aureus and Streptococcus faecalis: relation to antibacterial activity. Antimicrob Agents Chemother. 1980 Nov;18(5):834–836. doi: 10.1128/aac.18.5.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Liu F. Y. Penicillin-binding proteins in bacteria. Antimicrob Agents Chemother. 1980 Jul;18(1):148–157. doi: 10.1128/aac.18.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makover S. D., Wright R., Telep E. Penicillin-binding proteins in Haemophilus influenzae. Antimicrob Agents Chemother. 1981 Apr;19(4):584–588. doi: 10.1128/aac.19.4.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Datta N. Mutant drug resistant factors of high transmissibility. Nature. 1967 May 27;214(5091):885–887. doi: 10.1038/214885a0. [DOI] [PubMed] [Google Scholar]
- Mizuuchi K., O'Dea M. H., Gellert M. DNA gyrase: subunit structure and ATPase activity of the purified enzyme. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5960–5963. doi: 10.1073/pnas.75.12.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. Replication and repair of DNA in cells of Escherichia coli treated with toluene. Proc Natl Acad Sci U S A. 1970 Oct;67(2):674–681. doi: 10.1073/pnas.67.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y., Foulds J. Porin channels in Escherichia coli: studies with beta-lactams in intact cells. J Bacteriol. 1983 Jan;153(1):232–240. doi: 10.1128/jb.153.1.232-240.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Sykes R. B., Staniforth S. E. A new cephalosporin with a dual mode of action. Antimicrob Agents Chemother. 1976 Aug;10(2):245–248. doi: 10.1128/aac.10.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrini A. M., Geroldi D., Siccardi A., Falaschi A. Studies on the mode of action of nalidixic acid. Eur J Biochem. 1972 Feb 15;25(2):359–365. doi: 10.1111/j.1432-1033.1972.tb01704.x. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Clark D. C., Wotton S. Indirect method for assessing the penetration of beta-lactamase-nonsusceptible penicillins and cephalosporins in Escherichia coli strains. Antimicrob Agents Chemother. 1976 Aug;10(2):215–218. doi: 10.1128/aac.10.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu H., Nikaido H. Role of beta-lactam hydrolysis in the mechanism of resistance of a beta-lactamase-constitutive Enterobacter cloacae strain to expanded-spectrum beta-lactams. Antimicrob Agents Chemother. 1985 Mar;27(3):393–398. doi: 10.1128/aac.27.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]



