Abstract
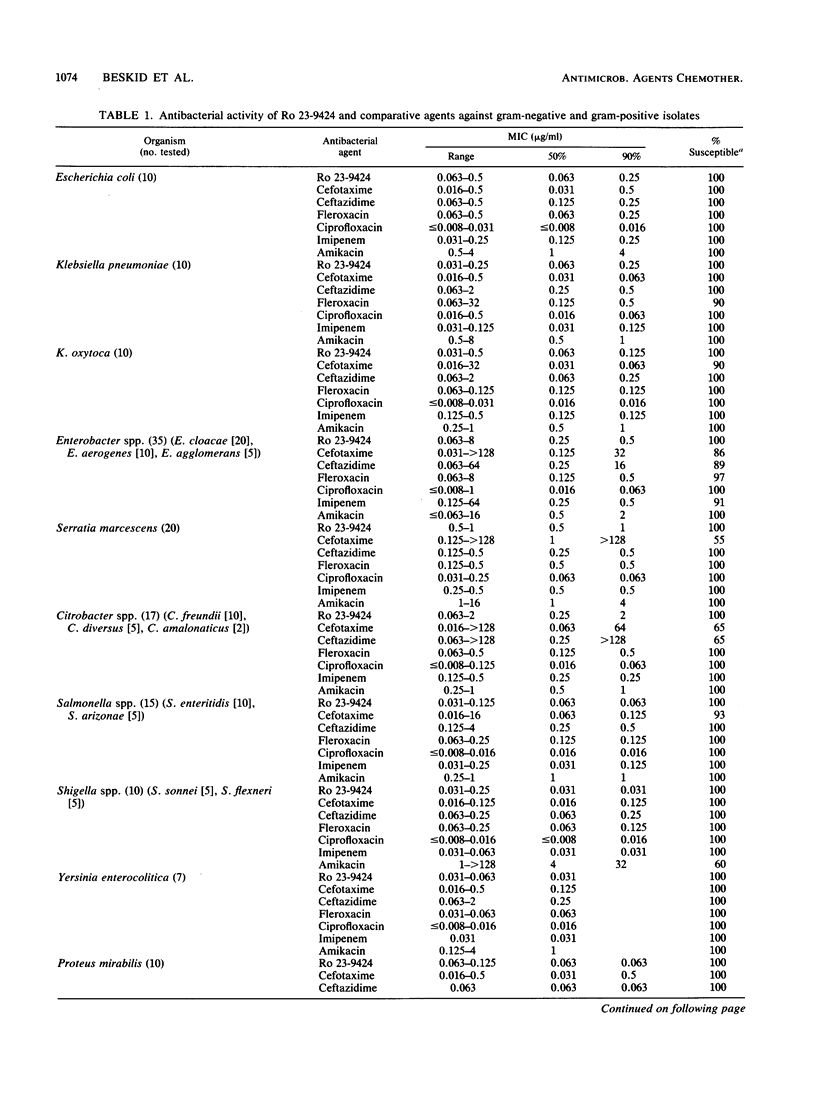
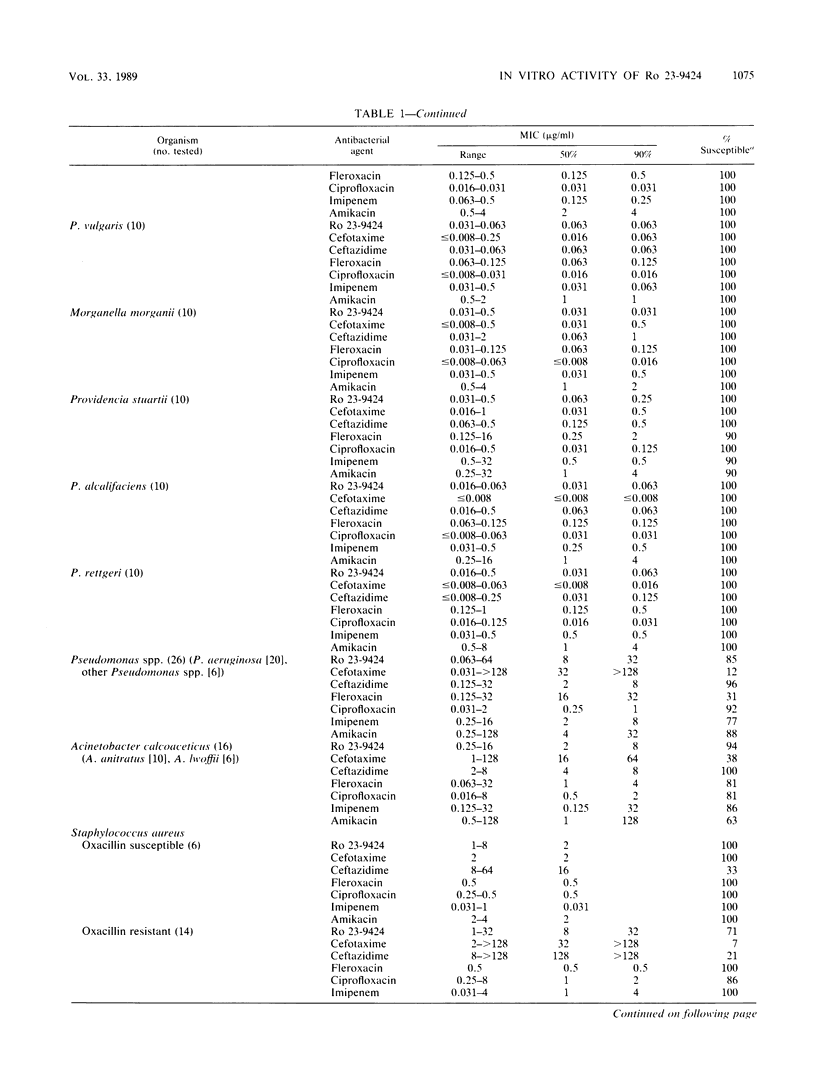
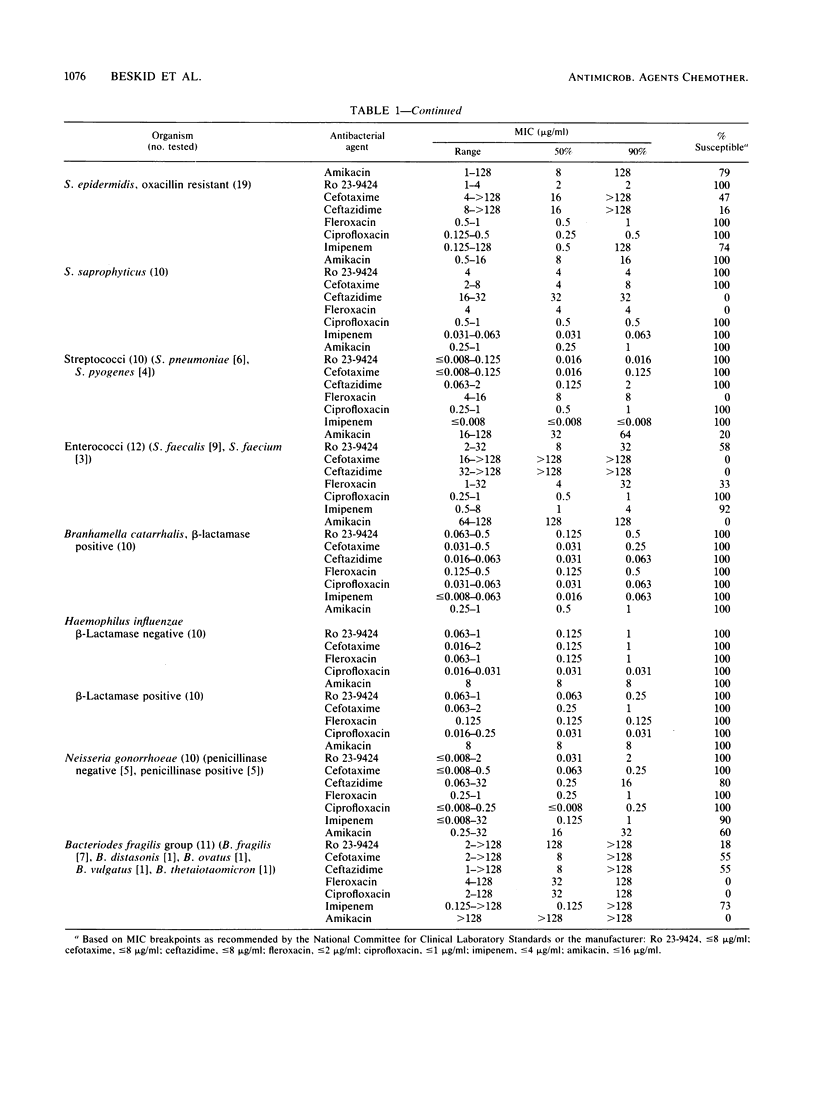
The in vitro activity of the dual-action antibacterial agent Ro 23-9424 was compared with those of cefotaxime, ceftazidime, ciprofloxacin, fleroxacin, imipenem, and amikacin against 358 aerobes and anaerobes. The MIC ranges, MICs for 50 and 90% of the strains (MIC50s and MIC90s), and percentage of strains susceptible for each agent at the recommended susceptible MIC breakpoint were determined for each genus. The MIC90s (micrograms per milliliter) of the agents against members of the family Enterobacteriaceae were as follows: ciprofloxacin, 0.063; Ro 23-9424, fleroxacin, and imipenem, 0.5; ceftazidime, 2; amikacin, 4; and cefotaxime, 16. The MIC90s (micrograms per milliliter) against Pseudomonas and Acinetobacter spp. were as follows: ciprofloxacin, 2; ceftazidime and imipenem, 8; Ro 23-9424, 16; fleroxacin, 32; amikacin, 64; and cefotaxime, 128. Against gram-positive bacteria, excluding the enterococci, the MIC90s (micrograms per milliliter) were as follows: ciprofloxacin, 1; imipenem, 4; Ro 23-9424 and fleroxacin, 8; amikacin, 64; and ceftazidime and cefotaxime, greater than 128. Against gram-positive bacteria, including the enterococci, the MIC90s changed only for the following agents: Ro 23-9424, 16 micrograms/ml; and amikacin, 128 micrograms/ml. Strains of Branhamella catarrhalis, Haemophilus influenzae, and Neisseria gonorrhoeae were 100% susceptible to Ro 23-9424, cefotaxime, ciprofloxacin, and fleroxacin, while the other three agents showed somewhat less activity only against N. gonorrhoeae. Against anaerobes, imipenem was the most effective agent, while the activities of the other six agents were variable.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boisvert W., Cheung K. S., Lerner S. A., Johnston M. Mechanisms of action of chloroalanyl antibacterial peptides. Identification of the intracellular enzymes inactivated on treatment of Escherichia coli JSR-O with the dipeptide beta Cl-LAla-beta Cl-LAla. J Biol Chem. 1986 Jun 15;261(17):7871–7878. [PubMed] [Google Scholar]
- Georgopapadakou N. H., Bertasso A., Chan K. K., Chapman J. S., Cleeland R., Cummings L. M., Dix B. A., Keith D. D. Mode of action of the dual-action cephalosporin Ro 23-9424. Antimicrob Agents Chemother. 1989 Jul;33(7):1067–1071. doi: 10.1128/aac.33.7.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood D., O'Grady F. Dual-action cephalosporin utilizing a novel therapeutic principle. Antimicrob Agents Chemother. 1976 Aug;10(2):249–252. doi: 10.1128/aac.10.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobashery S., Johnston M. Inactivation of alanine racemase by beta-chloro-L-alanine released enzymatically from amino acid and peptide C10-esters of deacetylcephalothin. Biochemistry. 1987 Sep 8;26(18):5878–5884. doi: 10.1021/bi00392a045. [DOI] [PubMed] [Google Scholar]
- Mobashery S., Johnston M. Reactions of Escherichia coli TEM beta-lactamase with cephalothin and with C10-dipeptidyl cephalosporin esters. J Biol Chem. 1986 Jun 15;261(17):7879–7887. [PubMed] [Google Scholar]
- O'Callaghan C. H., Sykes R. B., Staniforth S. E. A new cephalosporin with a dual mode of action. Antimicrob Agents Chemother. 1976 Aug;10(2):245–248. doi: 10.1128/aac.10.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]


