Abstract
Interleukin-12 (IL-12) is a pivotal cytokine in driving the immune system towards a T helper (Th)1 type response and preventing a Th2 type immune profile. Therefore, IL-12 is indispensable in the defense against certain, mainly intracellular pathogens, but overproduction of this cytokine is crucially involved in the etiology of several inflammatory and autoimmune diseases.
Hence, IL-12 is an ideal target for pharmacological intervention in the therapy of autoimmune and inflammatory diseases.
The production of IL-12 and a resultant Th1 type immune response can be suppressed with several pharmacological approaches including modulation of intracellular cyclic AMP levels, glucocorticoids and nuclear factor-κB inhibition. IL-12 responsiveness may be inhibited using anti-IL-12 antibodies, soluble IL-12 receptors or the IL-12 p40 homodimer.
Exploitation of these approaches may provide novel means for the experimental therapy of a variety of pathophysiological states.
Keywords: Inflammation, cytokines, autoimmune disease, interferon-γ, interleukin-2, interleukin-10, interleukin-4, transcription factors, catecholamine, nitric oxide
Introduction
Interleukin (IL)-12 is a heterodimeric cytokine that is secreted mainly by antigen-presenting cells and plays a key role in determining the nature of immune response to exogenous or endogenous antigens. IL-12 is comprised of two disulphide-linked protein subunits designated p35 and p40, which are encoded by two different genes (Trinchieri, 1995; Gately et al., 1998). Both subunits have to be produced within the same cell to obtain the biologically active dimer designated p70. The production of p40 exceeds the production of p70 by from 40 fold to more than 500 fold depending on the experimental system (Wysocka et al., 1995; Snijders et al., 1996; Haskó et al., 1998f). Five to forty per cent of this is secreted as a homodimer called p(40)2. The p(40)2 homodimer has been shown to exert antagonistic activity on the IL-12 receptor in both in vitro (Gilessen et al., 1995; Ling et al., 1995) and in vivo (Heinzel et al., 1997; Mattner et al., 1997; Rothe et al., 1997) systems. On the other hand, the p(40)2 homodimer stimulates the differentiation of CD8+ T cells with type 1 cytokine profile demonstrating agonistic properties (Gateley et al., 1998). The p35 subunit lacks any biological activity.
Cellular sources, inducers and cytokine regulators of IL-12
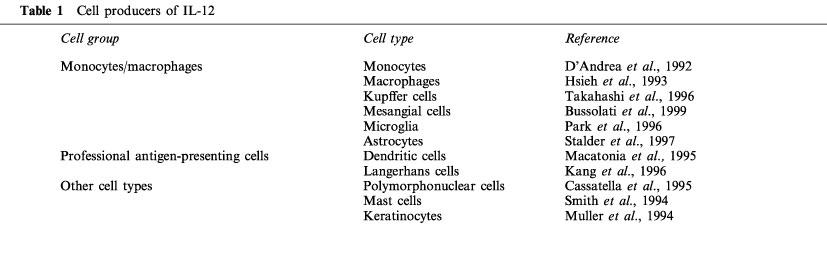
Although IL-12 was originally identified and purifed from supernatants of an Epstein-Barr virus-transformed human B lymphoblastoid cell line (Kobayashi et al., 1989), it is now clear that the main producers of IL-12 are cells of the innate immune system and professional antigen-presenting cells (Table 1). Cells of the monocyte/macrophage lineage including monocytes (D'Andrea et al., 1992), macrophages (Hsieh et al., 1993), Kupffer cells (Takahashi et al., 1996), mesangial cells (Bussolati et al., 1999) and glial cells (Park & Shin, 1996; Stalder et al., 1997) have all been shown to secrete IL-12. Other cell types of the innate system such as polymorphonuclear leukocytes (Cassatella et al., 1995) and mast cells (Smith et al., 1994), as well as keratinocytes (Muller et al., 1994) also express IL-12. Dendritic cells (Macatonia et al., 1995; Sousa et al., 1997) and Langerhans cells (Kang et al., 1996) are the most important professional antigen-presenting cells that produce IL-12.
Table 1.
Cell producers of IL-12
The production of IL-12 both in macrophages and dendritic cells can be induced by two different mechanisms (Figure 1). The first pathway involves the interaction of the producer cells with various micro-organisms or microbial products. Live intracellular bacteria, such as Listeria monocytogenes (Tripp et al., 1993) and various Mycobacteria (Zhang et al., 1994) are potent triggers for IL-12 production. Intracellular parasites including Leishmania major (Reiner et al., 1994) and Toxoplasma gondii (Gazzinelli et al., 1993) and viruses (Biron, 1997) are also important inducers of IL-12. Bacterial lipopolysaccharide (LPS; Baron et al., 1993), killed Mycobacterium tuberculosis (D'Andrea et al., 1992), bacterial superantigens such as staphylococcus enteroxin B (Haskó et al., 1998f), and unmethylated CpG nucleotides (Klinman et al., 1996) have all been reported to evoke IL-12 production. The other main mechanism of IL-12 induction involves stimulation of CD40 on antigen-presenting cells wtih membrane bound or soluble CD40 ligand during T lymphocyte-antigen-presenting cell interactions (Shu et al., 1995; Figure 1).
Figure 1.
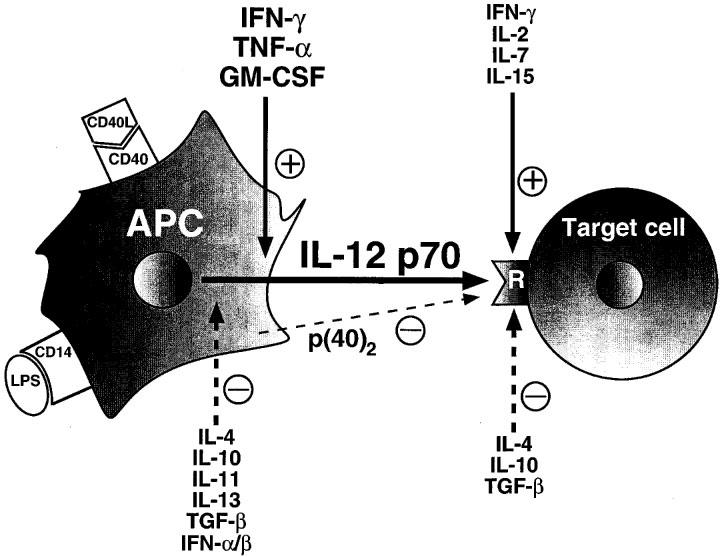
Schematic representation of the cytokine pathways involved in the modulation of IL-12 production and IL-12 receptor expression. The production of IL-12 in antigen presenting cells is induced by stimulation of CD40 by CD40 ligand or activation of CD14 by LPS. The production of IL-12 is subject to inhibition by IL-4, IL-10, IL-11, IL-13, TGF-β and IFN-α/β. IFN-γ, TNF-α and GM-CSF augment IL-12 release. IL-12 receptor expression is down-regulated by IL-4, IL-10 and TGF-β, and up-regulated by IFN-γ, IL-2, IL-7 and IL-15. Continuous lines represent stimulatory effects, while dotted lines represent inhibitory effects. The homodimer p(40)2 is a natural antagonist of the IL-12 receptor on Th cells. Abbreviations: APC, antigen-presenting cell; CD40L, CD40 ligand; LPS, lipopolysaccharide; R, IL-12 receptor.
The production of IL-12 is tightly regulated by other members of the cytokine cascade including both positive and negative regulatory signals (Figure 1). The most important positive regulators of IL-12 production are interferon (IFN)-γ (Ma et al., 1996), tumour necrosis factor (TNF)-α (Flesch et al., 1995) and granulocyte-monocyte colony stimulating factor (GM-CSF; Hayes et al., 1995; Randow et al., 1997). The potentially dangerous positive autoregulatory loop consisting of IL-12 and IFN-γ (see below) is offset by negative regulators of IL-12 production, such as IL-10 (D'Andrea et al., 1993; Berg et al., 1995), IL-4 (Snijders et al., 1996), IL-11 (Trepicchio et al., 1996), IL-13 (D'Andrea et al., 1995), transforming growth factor-β (TGF-β; D'Andrea et al., 1995) and IFN-α/β (Cousens et al., 1997). Interestingly, one of the mechanisms by which the human immunodeficiency virus (HIV) suppresses IL-12 production involves an indirect effect via induction of IL-10 by HIV-gp120 (Taoufik et al., 1997).
Cellular targets of IL-12 action
The main targets of IL-12 are T lymphocytes and natural killer (NK) cells (Trinchieri, 1995), but IL-12 also affects the function of B lymphocytes (Metzger et al., 1995) and hematopoietic progenitor cells (Jacobsen, 1995). IL-12 is an important link between innate and adaptive immunity, as it is secreted upon stimulation of antigen-presenting cells and activates IFN-γ production, proliferation, and cytolytic activity of NK cells and T lymphocytes (Trinchieri, 1995). In turn, IFN-γ has been shown to promote IL-12 production and macrophage activation, which provides the basis of an autoregulatory positive feedback loop resulting in a strong immune/inflammatory response directed against the antigen. Early in the immune response, IL-12 also plays a critical role in directing the development of T helper (Th)1 versus Th2 cell differentiation characterized by an increased production of IFN-γ and IL-2 (Th1 cytokines) and suppression of IL-4, IL-5 and IL-10 (Th2 cytokines) formation (Trinchieri, 1995). While a Th1 type immune response (cellular immunity) is crucial in the successful elimination of intracellular pathogens such as certain bacteria and viruses, and tumour cells, a Th2 response (humoral immunity) is critical for the removal of certain parasites, such as intestinal worms.
IL-12 receptors and signal transduction
IL-12 exerts its effects by binding to specific cell surface receptors on its target cells. The high affinity IL-12 receptor is formed by the coexpression of two subunits, the IL-12Rβ1 (Chua et al., 1994) and IL-12Rβ2 (Presky et al., 1996). While both the IL-12Rβ1 and IL-12Rβ2 are responsible for providing the binding energy, the IL-12Rβ2 is essential for signal transduction (Gately et al., 1998). The expression of IL-12Rβ2 appears to be confined to Th1 cells (Rogge et al., 1997; Szabo et al., 1997b), which may provide a selective therapeutic target for altering the Th1/Th2 balance in immunopathological conditions. Similar to the production of IL-12, the expression of both IL-12Rβ1 and IL-12Rβ2 is regulated by cytokines (Figure 1). While IL-2, IL-7, IL-15 and IFN-γ enhance IL-12 receptor expression, IL-4, IL-10 and TGF-β down-regulate IL-12 receptors and IL-12 responsiveness (Gollob et al., 1997; Rogge et al., 1997; Szabo et al., 1997b; Wu et al., 1997; Himmelreich et al., 1998).
IL-12 receptor stimulation in target cells activates the receptor-associated tyrosine kinases JAK2 and TYK2 and the transcription factors STAT3 and STAT4 (Bacon et al., 1995; Jacobson et al., 1995). Inhibition of tyrosine kinase activation may therefore provide a means for suppressing IL-12 responsiveness (see below).
Role of endogenous IL-12 in immunopathology
Autoimmune diseases are characterized by specific alterations in the expression of the above inflammatory mediators. In some of these diseases, a clear polarization of cytokine expression can be observed (Del Prete, 1998), either to the Th1 (multiple sclerosis, experimental allergic encephalomyelitis, autoimmune thyroid disease, and insulin-dependent diabetes mellitus) or to the Th2 direction (systemic lupus erythematosus, autoimmune hemolytic anaemia). In a Th1 cytokine response IFN-γ, IL-2, and TNF-α are the key players, while a Th2 profile is often associated with increased expression of IL-10, IL-4, IL-13 and TGF-β. Consequently, an approach to inhibit Th1 cytokines and potentiate Th2 cytokines would be desirable in Th1 diseases, and vice versa in Th2-mediated disorders. Because IL-12 is one of the central factors deciding the fate of the immune response concerning the polarity of this response (the presence of IL-12 shifts the balance towards a Th1 phenotype), this cytokine can be an ideal target for shaping the immune processes during autoimmune diseases. This hypothesis is corroborated by the fact that IL-12 has been shown to be directly and prominently involved in the induction of the pathophysiology of several autoimmune diseases including multiple sclerosis (Leonard et al., 1995), inflammatory bowel disease (Neurath et al., 1995), insulin dependent diabetes mellitus (Trembleau et al., 1995), glomerulonephritis (Kitching et al., 1999) and rheumatoid arthritis (Germann et al., 1995). The overproduction of IL-12 is also an important pathogenetic factor in inflammatory states such as septic shock (Wysocka et al., 1995) and the generalized Shwartzman reaction (Ozmen et al., 1994). Furthermore, a potential role for IL-12 was suggested in the promotion and maintenance of inflammation in atherosclerotic or psoriatic lesions (Uyemura et al., 1996; Yawalkar et al., 1998).
In contrast to the immunopathological role of overexpression of IL-12 in Th1 driven responses, IL-12 deficiency can contribute to an overactive Th2 type immune phenotype. This was best shown by the fact that IL-12 treatment reversed the airway hyperresponsiveness and decreased IL-4 and IL-5 expression in a murine model of asthma, a disease associated with a hyperreactive Th2 immune response (Gavett et al., 1995). IL-12 deficiency has been associated with tumour growth, while this cytokine has been successfully administered in patients with cancer (Lotze et al., 1996). Finally, treatment with IL-12 has been proposed for controlling viral infections such as chronic hepatitis or AIDS (Gately, 1997).
Anticytokine strategies based on immmunoneutralization of IL-12 and antagonism of IL-12 receptors
The recent success of anti-TNF-α antibodies (Elliott et al., 1994) and soluble TNF receptors (Moreland et al., 1997) in the short-term treatment of human rheumatoid arthritis suggest that strategies aiming at neutralizing other cytokines involved in the etiology of autoimmune diseases may be worth pursuing. The pivotal role of IL-12 in animal models of arthritis and other Th1-driven autoimmune diseases (see above) suggests that targeting this cytokine may be of therapeutical utility in these disease states. Besides similar approaches as in the case of anti-TNF-α therapies (anti-cytokine antibodies or soluble cytokine receptors), the use of the p(40)2 homodimer could constitute a different approach, based on the recent data that this molecule is a physiological antagonist of the IL-12 receptor (see above). In fact, exogenous application of IL-12 p(40)2 ameliorates the course of disease in animal models of insulin-dependent diabetes mellitus (Rothe et al., 1997) and endotoxaemia (Mattner et al., 1997).
Although such approaches have some promise, the long-term administration of these proteins during chronic autoimmune diseases can precipitate an immune response against these molecules resulting in the loss of efficacy and development of immune-complex disease. Another limitation of such drugs is that they should be administered parenterally. Therefore, it is important to identify small molecular weight compounds, which can regulate the production and/or activity of cytokines without the side effects of protein therapies. Recent studies have shown that the production and/or activity of IL-12 and consequently the balance between Th1 and Th2 responses can be modulated using small molecular weight compounds, which can interfere with the synthesis or action of this cytokine.
Pharmacological modulation of IL-12 production
The induction of IL-12 production is a highly regulated process involving multiple pathways, which provides several targets for modulation by small molecular weight compounds (Figure 2). The most important targets can be classified as follows: the cyclic AMP-protein kinase system, the transcription factor nuclear factor κB (NF-κB), the glucocorticoid receptor, cell membrane ion channels and pumps, and nitric oxide synthase. There are several other compounds, which do not fall into any of these categories, and will be discussed separately.
Figure 2.
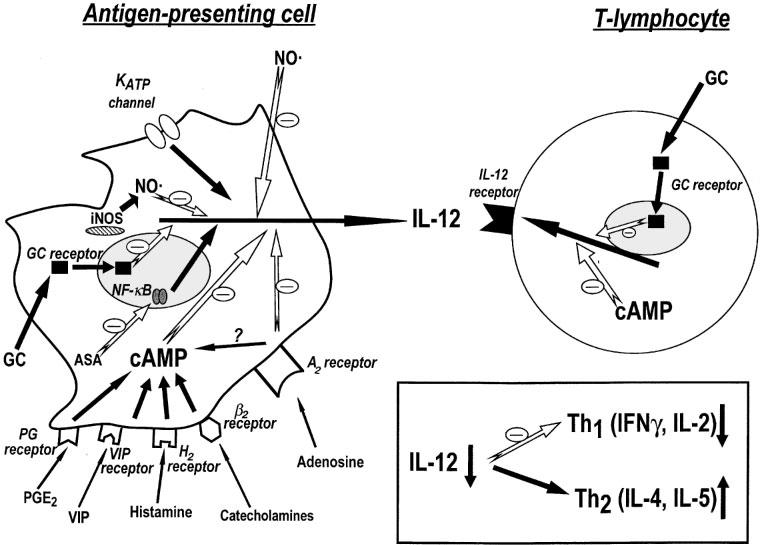
Schematic representation of the pathways involved in the modulation of IL-12 production and IL-12 receptor expression. IL-12 production can be inhibited by various approaches including elevation of intracellular cyclic AMP levels, activation of glucocorticoid receptors, inhibition of KATP channels, or inhibition of NF-κB. KATP channel activation enhances IL-12 production. The suppression of IL-12 shifts the immune response towards a T helper 2 profile, with decreased production of IFN-γ and IL-2, and augmented production of IL-4 and IL-5. Abbreviations: NO, nitric oxide; iNOS, inducible nitric oxide synthase; GC receptor, glucocorticoid receptor; ASA, acetyl salicylic acid; VIP, vasoactive intestinal peptide; NF-κB, nuclear factor κB; PG, prostaglandin.
Cyclic AMP modulating strategies
Considerable evidence suggests that alterations in intracellular cyclic nucleotide concentrations have a profound effect on cytokine production by immune/inflammatory cells (Pastores et al., 1996; Haskó & Szabó, 1998; Haskó et al., 1998b). Van der Pouw Kraan et al. (1995) showed for the first time using human monocytes that prostaglandin E2 (PGE2), an agent known to elevate intracellular cyclic AMP levels, potently suppresses IL-12 production. Other agents capable of increasing cyclic AMP levels, including the non-selective phosphodiesterase (PDE) inhibitor 3-isobutyl-1-methylxanthine and the cyclic AMP analogue N-2-O-dibutyryl-cyclic AMP mimicked the effect of PGE2. In another in vitro study, PGE2 was shown to inhibit IL-12 production in microglial cells (Levi et al., 1998). Recently, these results were confirmed in vivo, where intraperitoneal injection into mice of PGE2 prevented the systemic production of IL-12 in response to Escherichia coli infection (Takano et al., 1998). Consistent with the role of IL-12 in inducing a strong protective Th1 type immune response against bacteria, the resolution of infection was hampered in the PGE2-treated mice.
The predominant form of cyclic AMP PDE in monocytes/macrophages is the PDE IV isotype, but lower amounts of PDE III and PDE I can also be found in these cells (Verghese et al., 1995; Souness et al., 1996; Gantner et al., 1997; Kelly et al., 1998). Selective inhibition of these enzymes suppresses IL-12 production in various in vivo models. For example, treatment of endotoxemic mice with the PDE IV inhibitor rolipram or the PDE III blocker amrinone suppressed plasma IL-12 levels and consequently decreased mortality (Haskó et al., 1998e). Rolipram also suppressed IL-12 production, down-regulated the on-going Th1 response and ameliorated the course of collagen-induced arthritis, autoimmune diabetes, and experimental allergic encephalomyelitis in both rodent and primate models (Genain et al., 1995; Sommer et al., 1995; Ross et al., 1997; Liang et al., 1998). The non-selective phosphodiesterase inhibitor pentoxifylline was evaluated in human patients with multiple sclerosis and was found to inhibit IL-12 production by peripheral blood mononuclear cells (Rieckmann et al., 1996). The suppressed IL-12 levels coincided with a deviation towards a Th2 type cytokine profile and six out of eight patients reported improved motor skills and less fatigue.
Besides PGE2, other endogenous molecules which elevate cyclic AMP can also down-regulate IL-12 production. Most notably, the catecholamines adrenaline and noradrenaline are potent inhibitors of IL-12 release in human whole blood (Elenkov et al., 1996). These stress hormones act through β2-adrenoceptors located on monocytes and dendritic cells and the mechanism of action involves transcriptional inhibition of both the p35 and p40 subunits and posttranslational inhibition of p35 (Panina-Bordignon et al., 1997). Again, the inhibition of IL-12 production coincided with a shift towards a Th2 type immune response. These observations might have important implications for the treatment of asthma, because the beneficial bronchodilatory effects of β2-adrenoceptor agonists can, in the long term, be overshadowed by their ability to skew the immune response towards a harmful Th2 direction. The above in vitro findings were confirmed in endotoxemic mice, where isoproterenol, a non-selective agonist of β-adrenoceptors blunted the plasma IL-12 response (Haskó et al., 1998d). In contrast, the same study demonstrated that propanolol, a β-adrenoceptor antagonist augmented the release of this cytokine indicating that endogenously released catecholamines are involved in dampening the potentially harmful cytokine burst in acute systemic inflammation. Because septic shock is caused by an uncontrollable release of cytokines such as IL-12 and TNF-α, the potential immunomodulatory effects of β-adrenoceptor stimulation should also be considered during catecholamine treatment of septic states.
The purine adenosine is another endogenous agent capable of inhibiting both IL-12 production and Th1 development (Haskó et al., unpublished observation). Our results obtained with selective adenosine receptor agonists and antagonists suggest that the inhibition of IL-12 production is mainly dependent on A2a receptor activation (Haskó et al., unpublished observation). On the other hand, the suppression of TNF-α by adenosine is primarily due to A3 receptor activation (McWhinney et al., 1996; Sajjadi et al., 1996). Because both A2a (Ralevic & Burnstock, 1998) and A3 (Hines et al., 1999) receptors can be positively coupled to cyclic AMP, it can be suggested that the adenosine inhibition of both IL-12 and TNF-α is due to elevation of intracellular cyclic AMP levels. In agreement with the above in vitro data, the A3 receptor agonist N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide (IB-MECA) suppresses IL-12 production in both arthritic and endotoxaemic mice, which correlates with its beneficial effect in both animal models (Haskó et al., 1998a; Szabó et al., 1998).
A whole host of endogenous molecules known to stimulate cyclic AMP, such as histamine, calcitonin gene-related peptide, vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating peptide have been shown to inhibit IL-12 release in various in vitro experimental systems (Fox et al., 1997; Dewit et al., 1998; Van der Pouw Kraan et al., 1998; Xin & Sriram, 1998). Whether the endogenous production of these mediators affects IL-12 production, remains to be clarified.
Glucocorticoids
Glucocorticoids are used therapeutically as potent anti-inflammatory and immunosuppressive agents for a wide range of diseases, including autoimmune diseases, allergic states and other inflammatory illnesses. It has been known for a long time that these hormones are potent inhibitors of proinflammatory cytokine production, which is now considered as one of the central mechanisms of their anti-inflammatory action (Wilckens & De Rijk, 1997). Recently, it has been demonstrated that glucocorticoid hormones are important regulators of IL-12 production. Elenkov et al. (1996) showed for the first time that dexamethasone inhibits bioactive IL-12 production in LPS-stimulated human whole blood, an effect, which is effectively antagonized with a glucocorticoid receptor antagonist. Subsequently, it was shown that glucocorticoids inhibit IL-12 release from both monocytes and macrophages, as well as dendritic cells, and the reduced IL-12 production in all cases decreases the capacity of these cells to drive a Th1 response but enhances their ability to direct Th2 cytokine production (Blotta et al., 1997; DeKruyff et al., 1998; Vieira et al., 1998). Since dendritic cell IL-12 production is more important in the initiation of immune processes while macrophage-derived IL-12 is more significant in the maintenance of immune activation (Sousa et al., 1997), glucocorticoid suppression of IL-12 assures a continuous immunosuppressive effect. However, similarly to β2-adrenoceptor agonists, chronic treatment of allergic diseases with corticosteroids may exacerbate the course of disease, because it promotes the overproduction of Th2 cytokines, which are already elevated and are causative factors in the pathogenesis of asthma and other allergic diseases. Recently, these in vitro findings were reproduced in an in vivo system, where treatment of mice with dexamethasone potently suppressed LPS-induced plasma IL-12 concentrations (Haskó et al., 1998d). Furthermore, endogenously secreted glucocorticoids also decrease IL-12 production, illustrated by the fact that inducibility of IL-12 exhibits diurnal rhythmicity in LPS-stimulated human whole blood; when plasma cortisol levels are low, the secretion of IL-12 is enhanced, but high plasma cortisol is paralleled with lower IL-12 release (Petrovsky et al., 1998). Interestingly, the HIV accessory gene product Vpr decreases IL-12 production and promotes the spread of infection by mimicking the effect of glucocorticoids via triggering the glucocorticoid receptor complex (Ayyavoo et al., 1997).
Targeting NF-κB
The transcription factor NF-κB plays a central role in coordinating immune/inflammatory processes. Recent studies have identified putative NF-κB sites in the promoter regions of both the IL-12 p35 and p40 genes (Murphy et al., 1995; Plevy et al., 1997). Recently, pharmacological evidence has been obtained that inhibition of this transcription factor system can decrease IL-12 production. For example, pyrrolidine dithiocarbamate, a selective blocker of NF-κB has been shown to inhibit LPS-induced IL-12 production both in vivo (Németh et al., 1998a) and in vitro (Haskó et al., unpublished observation). The in vivo inhibition of IL-12 by pyrrolidine dithiocarbamate exerts a protective effect against endotoxemic shock in mice (Németh et al., 1998a). This study also showed that pyrrolidine dithiocarbamate increases the production of IL-10, indicating that the inhibitory effect of this agent is selective for proinflammatory cytokines. Interestingly, the steroid hormone 1,25-dihydroxyvitamin D3 suppressed IL-12 production not by binding to the vitamin D3 receptor, but via inhibition of NF-κB binding to its consensus sequence on the IL-12 p40 gene (D'Ambrosio et al., 1998). 1,25-dihydroxyvitamin D3 suppresses Th1 cytokine production and protects against diabetes in the IL-12 dependent non-obese diabetic mice (Casteels et al., 1998).
Similarly to 1,25-dihydroxyvitamin D3, acetyl salicylic acid suppresses IL-12 production and Th1 development by a mechanism involving decreased NF-κB activation (Mazzeo et al., 1998). The Vpr-induced repression of IL-12 production (see above) also involves NF-κB (Ayyavoo et al., 1997). Although direct evidence has not yet been presented, it is conceivable that glucocorticoids, which are known to inhibit NF-κB (Dumont et al., 1998), may suppress IL-12 by interaction with this transcription factor system. Finally, retinoids inhibit IL-12 production by forming a transcriptionally inhibitory complex with NF-κB (Na et al., 1999).
Ion channels and pumps
The movement of ions across cell membranes mediates several cellular processes in the immune system and there is a large body of evidence indicating that altering the activity of ion channels and pumps can profoundly affect cytokine production (Haslberger et al., 1992; Hamon et al., 1997; Szabó et al., 1997a; Haskó et al., 1998c). IL-12 production is also subject to modulation by changes in ion movements. Blockade of dihydropyridine-sensitive calcium channels inhibits IL-12 production in human dendritic cells, which can be prevented by a calcium channel agonist (Poggi et al., 1998). These channels are also the molecular targets of HIV Tat, which blocks both calcium influx and IL-12 release in the dendritic cells. This mechanism might contribute to the immunosuppression seen during HIV infection. In LPS-treated mice, the calcium channel antagonists verapamil and diltiazem are unable to suppress plasma IL-12 levels, however, dantrolene, an agent known to prevent the release of calcium from intracellular stores inhibits IL-12 production (Németh et al., 1998b). Our group recently demonstrated that by modulating ATP-gated K+ channels on immune cells, IL-12 production can be altered substantially: glibenclamide, a selective inhibitor of this channel, potently inhibits the release of this cytokine, while diazoxide, an opener of this channel, considerably increases IL-12 secretion (Haskó et al., unpublished observations). Consequently, in anti-CD3-stimulated mouse spleen cells, glibenclamide decreases the production of Th1 cytokines, but augments the production of the Th2 cytokine IL-4. Finally, inhibition of the Na/H antiporter by amiloride decreases IL-12 production, without altering the Th1/Th2 ratio: both IL-4 and IFN-γ production are inhibited by this agent in anti-CD3 antibody stimulated spleen cells (Haskó et al., unpublished observations).
Nitric oxide
The role of nitric oxide in the modulation of IL-12 expression is controversial. In the study of Rothe et al. (1996), addition of the nitric oxide synthase inhibitor N(G)-methyl-L-arginine suppressed INF-γ-induced IL-12 p40 mRNA formation, while nitric oxide generating compounds induced p40 mRNA. Furthermore, inhibition of nitric oxide synthase in mice with both N(G)-nitro-L-arginine-methylester and aminoguanidine decreased IL-12 production by dispersed lung cell cultures (Hogaboam et al., 1997; 1998). On the other hand, in J774 macrophages, N(G)-methyl-L-arginine markedly enhanced IL-12 protein secretion, while nitric oxide-generating compounds decreased it (Huang et al., 1998). Also, a number of recent studies showed that mice deficient in the inducible nitric oxide synthase produce enhanced amounts of IL-12 when compared to their heterozygous or wild-type counterparts (Huang et al., 1998; MacLean et al., 1998).
Immunosuppressive agents
Some of the most widely used immunosuppressive agents turned out to be potent inhibitors of IL-12 production. For example, tacrolimus (FK 506), a drug primarily used in organ transplantation, decreases IL-12 expression during primary skin responses (Homey et al., 1998). Thalidomide, which has beneficial effects in a host of inflammatory diseases, is a potent inhibitor of IL-12 production, and exerts its affect by a post-transcriptional mechanism (Moller et al., 1997). In contrast, cyclosporin A enhances IL-12 production in mouse spleen cells induced by CpG oligonucleotides (Redford et al., 1998), however, it remains to be determined whether this is the case when other inflammatory or immune stimuli are used. Other agents that are known to have immunosuppressive effects such as angiotensin converting enzyme inhibitors (Constantinescu et al., 1998) or sulfasalazine (Haskó et al., unpublished observation) are also capable of suppressing IL-12 production.
Modulation of IL-12 responsiveness
The expression of both subunits of the IL-12 receptor is up-regulated upon cellular activation. There is only very limited information available on the pharmacological modulation of the IL-12 receptor. In a recent study using human peripheral blood mononuclear cells and T lymphocytes, it has been shown that the expression of both chains of the IL-12 receptor is subject to suppression by dexamethasone or PGE2 (Wu et al., 1998). Dibutyryl cyclic AMP, 8-Br-cyclic AMP or cholera toxin mimicks the effect of PGE2 suggesting that it mediates its effects through enhancement of cyclic AMP (Wu et al., 1998; Braun et al., 1999). In contrast to the above agents, lisofylline inhibits IL-12 responsiveness, however it fails to suppress IL-12 production (Bright et al., 1998). It remains to be determined whether the action of lisofylline is due to an effect on the expression of IL-12 receptor or an interference with the intracellular events triggered by IL-12/IL-12R interaction. IL-12 responsiveness has been shown to be inhibited by protein kinase C and tyrosine kinase inhibition (Gerosa et al., 1993; Ye et al., 1995).
Conclusions
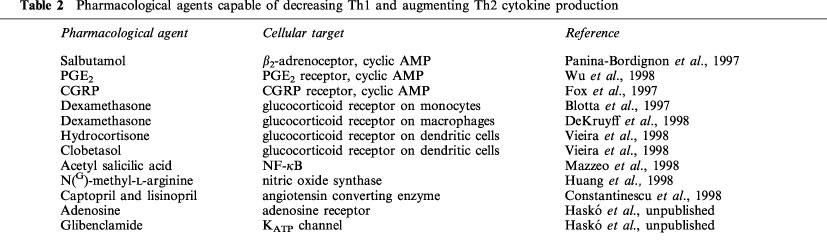
IL-12 plays an essential role in the protective immune responses against intracellular pathogens by directing the development of Th1 versus Th2 reactions. On the other hand, IL-12 driven Th1 reactions have deleterious consequences in certain autoimmune/inflammatory diseases. Therefore, the IL-12/IL-12 receptor system is an ideal target for pharmacological intervention in both immunodeficient states, when Th1 type immune responses are in demand or in ‘hyperimmune' diseases, when an ongoing Th1-type immune response underlies the pathophysiological processes. Recent studies have identified a number of pharmacological approaches, which are able to influence IL-12 production and Th1 versus Th2 immune responses (Table 2). It is clear that most of these approaches are not specific for IL-12 and also influence the production of other cytokines and mediators. Because in most inflammatory states, there is a high degree of redundancy between the function of proinflammatory cytokines, the ability of an agent to inhibit several interrelated proinflammatory pathways may even be desirable. Furthermore, in some cases, the inhibition of IL-12 by a compound is paralleled by elevated IL-10 production, which may result in an even stronger anti-inflammatory effect. On the other hand, it is conceivable that the non-selective blockade of proinflammatory pathways can be harmful in some pathophysiological states. For instance, when a chronic autoimmune process is complicated with an infectious disease, preservation of some immune functions may be crucial and selective pharmacological interventions to suppress the production of certain, but not all cytokines should be applied. In any case, exploitation of these approaches may provide novel means for the experimental therapy of a variety of pathophysiological states.
Table 2.
Pharmacological agents capable of decreasing Th1 and augmenting Th2 cytokine production
Abbreviations
- GM-CSF
granulocyte-monocyte colony stimulating factor
- HIV
human immunodeficiency virus
- IB-MECA
N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide
- IFN
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- NF-κB
nuclear factor κB
- NK
natural killer
- PDE
phosphodiesterase
- PGE2
prostaglandin E2
- TGF
transforming growth factor
- Th
T helper
- TNF
tumour necrosis factor
References
- AYYAVOO V., MAHBOUBI A., MAHALINGAM S., RAMALINGAM R., KUDCHODKAR S., WILLIAMS W.V., GREEN D.R., WEINER D.B. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor kappa B. Nat. Med. 1997;3:1117–1123. doi: 10.1038/nm1097-1117. [DOI] [PubMed] [Google Scholar]
- BACON C.M., MCVICAR D.W., ORTALDO J.R., REES R.C., O'SHEA J.J., JOHNSTON J.A. Interleukin 12 (IL-12) induces tyrosine phosphorylation of JAK2 and TYK2: differential use of Janus family tyrosine kinases by IL-2 and IL-12. J. Exp. Med. 1995;181:399–404. doi: 10.1084/jem.181.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARON P., CONSTANTIN G., D'ANDREA A., PONZIN D., SCARPINI E., SCARLATO G., TRINCHIERI G., ROSSI F., CASSATELLA M.A. Production of tumor necrosis factor and other proinflammatory cytokines by human mononuclear phagocytes stimulated with myelin P2 protein. Proc. Natl. Acad. Sci. U.S.A. 1993;90:4414–4418. doi: 10.1073/pnas.90.10.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERG D.J., KÜHN R., RAJEWSKY K., MÜLLER W., MENON S., DAVIDSON N., GRÜNIG G., RENNICK D. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J. Clin. Invest. 1995;96:2339–2347. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRON C.A. Activation and function of natural killer cell responses during viral infections. Curr. Opin. Immunol. 1997;9:24–34. doi: 10.1016/s0952-7915(97)80155-0. [DOI] [PubMed] [Google Scholar]
- BLOTTA M.H., DEKRUYFF R.H., UMETSU D.T. Corticosteroids inhibit IL-12 production in human monocytes and enhance their capacity to induce IL-4 synthesis in CD4+ lymphocytes. J. Immunol. 1997;158:5589–5595. [PubMed] [Google Scholar]
- BRAUN M.C., HE J., WU C.Y., KELSALL B.L. Cholera toxin suppresses interleukin (IL)-12 production and IL-12 receptor beta1 and beta2 chain expression. J. Exp. Med. 1999;189:541–552. doi: 10.1084/jem.189.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIGHT J.J., DU C., COON M., SRIRAM S., KLAUS S.J. Prevention of experimental allergic encephalomyelitis via inhibition of IL-12 signaling and IL-12-mediated Th1 differentiation: an effect of the novel anti-inflammatory drug lisofylline. J. Immunol. 1998;161:7015–7022. [PubMed] [Google Scholar]
- BUSSOLATI B., MARIANO F., BIANCONE L., FOA R., DAVID S., CAMBI V., CAMUSSI G. Interleukin-12 is synthesized by mesangial cells and stimulates platelet-activating factor synthesis, cytoskeletal reorganization, and cell shape change. Am. J. Pathol. 1999;154:623–632. doi: 10.1016/S0002-9440(10)65307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASSATELLA M.A., MEDA L., GASPERINI S., D'ANDREA A., MA X., TRINCHIERI G. Interleukin-12 production by human polymorphonuclear leukocytes. Eur. J. Immunol. 1995;25:1–5. doi: 10.1002/eji.1830250102. [DOI] [PubMed] [Google Scholar]
- CASTEELS K., WAER M., BOUILLON R., DEPOVERE J., VALCKX D., LAUREYS J., MATHIEU C. 1,25-Dihydroxyvitamin D3 restores sensitivity to cyclophosphamide-induced apoptosis in non-obese diabetic (NOD) mice and protects against diabetes. Clin. Exp. Immunol. 1998;112:181–187. doi: 10.1046/j.1365-2249.1998.00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUA A.O., CHIZZONITE R., DESAI B.B., TRUITT T.P., NUNES P., MINETTI L.J., WARRIER R.R., PRESKY D.H., LEVINE J.F., GATELY M.K., GUBLER U. Expression cloning of a human IL-12 receptor component. A new member of the cytokine receptor superfamily with strong homology to gp130. J. Immunol. 1994;153:128–136. [PubMed] [Google Scholar]
- CONSTANTINESCU C.S., GOODMAN D.B., VENTURA E.S. Captopril and lisinopril suppress production of interleukin-12 by human peripheral blood mononuclear cells. Immun. Lett. 1998;62:25–31. doi: 10.1016/s0165-2478(98)00025-x. [DOI] [PubMed] [Google Scholar]
- COUSENS L.P., ORANGE J.S., SU H.C., BIRON C.A. Interferon-α/β inhibition of interleukine 12 and interferon-γ production in vitro and endogenously during viral infection. Proc. Natl. Acad. Sci. U.S.A. 1997;94:634–639. doi: 10.1073/pnas.94.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'AMBROSIO D., CIPITELLI M., COCCIOLO M.G., MAZZEO D., DI LUCIA P., LANG R., SINIGAGLIA F., PANINA-BORDIGNON P. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3: involvement of NF-κB downregulation in transcriptional repression of the p40 gene. J. Clin. Invest. 1998;101:252–262. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'ANDREA A., ASTE-AMEZAGA M., VALIANTE N.M., MA X., KUBIN M., TRINCHIERI G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'ANDREA A., MA X., ASTE-AMEXAGA M., PAGANIN C., TRINCHIERI G. Stimulatory and inhibitory effects of interleukin (IL)-4 and IL-13 on the production of cytokines by human peripheral blood mononuclear cells: priming for IL-12 and tumor necrosis factor α production. J. Exp. Med. 1995;181:537–546. doi: 10.1084/jem.181.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'ANDREA A., RENGARAJU M., VALIANTE N.M., CHEHIMI J., KUBIN M., ASTE M., CHAN S.H., KOBAYASHI M., YOUNG D., NICKBARG E., CHIZZONITE R., WOLF S.F., TRINCHIERI G. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J. Exp. Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEKRUYFF R.H., FANG Y., UMETSU D.T. Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. J. Immunol. 1998;160:2231–2237. [PubMed] [Google Scholar]
- DEL PRETE G. The concept of type-1 and type-2 helper T cells and their cytokines in humans. Int. Rev. Immunol. 1998;16:427–455. doi: 10.3109/08830189809043004. [DOI] [PubMed] [Google Scholar]
- DEWIT D., GOURLET P., AMRAOUI Z., VERTONGEN P., WILLEMS F., ROBBERECHT P., GOLDMAN M. The vasoactive intestinal peptide analogue RO25-1553 inhibits the production of TNF and IL-12 by LPS-activated monocytes. Immunol. Lett. 1998;60:57–60. doi: 10.1016/s0165-2478(97)00129-6. [DOI] [PubMed] [Google Scholar]
- DUMONT A., HEHNER S.P., SCHMITZ M.L. , GUSTAFSSON J.A., LIDEN J., OKRET S., VAN DER SAAG P.T., WISSINK S., VAN DER BURG B., HERRLICH P., HAEGEMAN G., DE BOSSCHER K., FIERS W. Cross-talk between steroids and NF-kappa B: what language. Trends Biochem. Sci. 1998;23:233–235. doi: 10.1016/s0968-0004(98)01212-2. [DOI] [PubMed] [Google Scholar]
- ELENKOV I.J., PAPANICOLAU D.A., WILDER R.L., CHROUSOS G.P. Modulatory effects of glucocorticoids and catecholamines on human interleukin-12 and interleukin-10 production: clinical implications. Proc. Assoc. Am. Physicians. 1996;108:1–8. [PubMed] [Google Scholar]
- ELLIOTT M.J., MAINI R.N., FELDMANN M., KALDEN J.R., ANTONI C., SMOLEN J.S., LEEB B., BREEDVELD F.C. , MACFARLANE J.D., BIJL H., WOODY J.N. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor α (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994;344:1105–1110. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- FLESCH I.E.A., HESS J.H., HUANG S., AGUET M., ROTHE J., BLUETHMANN H., KAUFMANN S.H.E. Early interleukin 12 production by macrophages in response to mycobacterial infection depends on interferon γ and tumor necrosis factor α. J. Exp. Med. 1995;181:1615–1621. doi: 10.1084/jem.181.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX F.E., KUBIN M., CASSIN M., NIU Z., HOSOI J., TORII H., GRANSTEIN R.D., TRINCHIERI G., ROOK A.H. Calcitonin gene-related peptide inhibits proliferation and antigen presentation by human peripheral blood mononuclear cells: effects on B7, interleukin 10, and interleukin 12. J. Invest. Dermatol. 1997;108:43–48. doi: 10.1111/1523-1747.ep12285627. [DOI] [PubMed] [Google Scholar]
- GANTNER F., KUPFERSCHMIDT R., SCHUDT C., WENDEL A., HATZELMANN A. In vitro differentiation of human monocytes to macrophages: change of PDE profile and its relationship to suppression of tumor necrosis factor-α release by PDE inhibitors. Br. J. Pharmacol. 1997;121:221–231. doi: 10.1038/sj.bjp.0701124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GATELY M.K. Interleukin-12: potential clinical applications in the treatment of chronic viral hepatitis. J. Viral. Hepat. 1997. pp. 33–39. [DOI] [PubMed]
- GATELY M.K., RENZETTI L.M., MAGRAM J., STERN A.S., ADORINI L., GUBLER U., PRESKY D.H. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu. Rev. Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- GAVETT S.H., O'HEARN D.J., LI X., HUANG S.K., FINKELMAN F.D., WILLS-KARP M. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J. Exp. Med. 1995;182:1527–1536. doi: 10.1084/jem.182.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAZZINELLI R.T., HIENY S., WYNN T.A., WOLF S., SHER A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon γ by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc. Natl. Acad. Sci. U.S.A. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENAIN C.P., ROBERTS T., DAVIS R.L., NGUYEN M.H., UCCELLI A., FAULDS D., LI Y., HEDGPETH J., HAUSER S.L. Prevention of autoimmune demyelination in non-human primates by a cAMP-specific phosphodiesterase inhibitor. Proc. Natl. Acad. Sci. U.S.A. 1995;92:3601–3605. doi: 10.1073/pnas.92.8.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERMANN T., SZELIGA J., HESS H., STÖRKEL J., PODLASKI F.J., GATELY M.K., SCHMITT E., RÜDE E. Administration of interleukin 12 in combination with type II collagen induces severe arthritis in DBA/1 mice. Proc. Natl. Acad. Sci. U.S.A. 1995;92:4823–4827. doi: 10.1073/pnas.92.11.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEROSA F., TOMMASI M., BENATI C., GANDINI G., LIBONATI M., TRIDENTE G., CARRA G., TRINCHIERI G. Differential effects of tyrosine kinase inhibition in CD69 antigen expression and lytic activity induced by rIL-2, rIL-12, and rIFN-α in human NK cells. Cell. Immunol. 1993;150:382–390. doi: 10.1006/cimm.1993.1206. [DOI] [PubMed] [Google Scholar]
- GILLESSEN S., CARVAJAL D., LING P., PODLASKI F.J., STREMLO D.L., FAMILLETTI P.C., GUBLER U., PRESKY D.H., STERN A.S., GATELY M.K. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur. J. Immunol. 1995;25:200–206. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- GOLLOB J.A., KAWASAKI H., RITZ J. Interferon-γ and interleukin-4 regulate T cell interleukin-12 responsiveness through the differential modulation of high-affinity interleukin-12 receptor expression. Eur. J. Immunol. 1997;27:647–652. doi: 10.1002/eji.1830270311. [DOI] [PubMed] [Google Scholar]
- HAMON Y., LUCIANI M.-F., BECQ F., VERRIER B., RUBARTELLI A., CHIMINI G. Interleukin-1β secretion is impaired by inhibitors of the Atp binding cassette transporter, ABC1. Blood. 1997;90:2911–2915. [PubMed] [Google Scholar]
- HASKÓ G., NÉMETH Z.H., VIZI E.S., SALMAN A.L., SZABÓ C. An agonist of A3 adenosine receptors decreases IL-12, IFN-γ, and nitric oxide production and prevents lethality in endotoxemic mice. Eur. J. Pharmacol. 1998a;358:261–268. doi: 10.1016/s0014-2999(98)00619-0. [DOI] [PubMed] [Google Scholar]
- HASKÓ G., SHANLEY T.P., EGNACZYK G., NÉMETH Z.H., SALZMAN A.L., VIZI E.S., SZABÓ C. Exogenous and endogenous catecholamines inhibit the production of macrophage inflammatory protein (MIP) 1α via a β adrenoceptor mediated mechanism. Br. J. Pharmacol. 1998b;125:1297–1304. doi: 10.1038/sj.bjp.0702179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASKÓ G., SZABÓ C. Regulation of cytokine and chemokine production by transmitters and co-transmitters of the autonomic nervous system. Biochem. Pharmacol. 1998;56:1079–1087. doi: 10.1016/s0006-2952(98)00153-1. [DOI] [PubMed] [Google Scholar]
- HASKÓ G., SZABÓ C., NÉMETH Z.H., LENDVAI B., VIZI E.S. Modulation by dantrolene of endotoxin-induced interleukin-10, tumor necrosis factor-α, and nitric oxide production in vivo and in vitro. Br. J. Pharmacol. 1998c;124:1099–1106. doi: 10.1038/sj.bjp.0701934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASKÓ G., SZABÓ C., NÉMETH Z.H., SALZMAN A.L., VIZI E.S. Stimulation of β-adrenoceptors inhibits endotoxin-induced IL-12 production in normal and IL-10 deficient mice. J. Neuroimmunol. 1998d;88:57–61. doi: 10.1016/s0165-5728(98)00073-3. [DOI] [PubMed] [Google Scholar]
- HASKÓ G., SZABÓ C., NÉMETH Z.H., SALZMAN A.L., VIZI E.S. Suppression of interleukin-12 production by phosphodiesterase inhibition in murine endotoxemia is interleukin-10 independent. Eur. J. Immunol. 1998e;28:468–472. doi: 10.1002/(SICI)1521-4141(199802)28:02<468::AID-IMMU468>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- HASKÓ G., VIRÁG L., EGNACZYK G., SALMAN A.L., SZABÓ C. The crucial role of IL-10 in the suppression of the immunological response in mice exposed to staphylococcal enterotoxin B. Eur. J. Immunol. 1998f;28:1417–1425. doi: 10.1002/(SICI)1521-4141(199804)28:04<1417::AID-IMMU1417>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- HASLBERGER A., ROMANIN C., KOERBER R. Membrane potential modulates release of tumor necrosis factor in lipopolysaccharide-stimulated mouse macrophages. Mol. Biol. Cell. 1992;3:451–460. doi: 10.1091/mbc.3.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYES M.P., WANG J., NORCROSS M.A. Regulation of interleukin-12 expression in human monocytes: selective priming by interferon-γ of lipopolysaccharide-inducible p35 and p40 genes. Blood. 1995;86:646–650. [PubMed] [Google Scholar]
- HEINZEL F.P., HUJER A.M., AHMED F.N., RERKO R.M. In vivo production and function of IL-12 p40 homodimers. J. Immunol. 1997;158:4381–4388. [PubMed] [Google Scholar]
- HIMMELRICH H., PARRA-LOPEZ C., TACCHINI-COTTIER F., LOUIS J.A., LAUNOIS P. The IL-4 rapidly produced in BALB/c mice after infection with Leishmania major down-regulates IL-12 receptor β2-chain expression on CD4+ T cells resulting in a state of unresponsiveness to IL-12. J. Immunol. 1998;161:6156–6163. [PubMed] [Google Scholar]
- HINES C.K., WALKER L.L., CHEN J.F., JACOBSON M.A., FINK J.S., SCHARZCHILD M.A., SALVATORE C.A., MARQUARDT D.L. Effects of A2a and A3 adenosine receptor ablation on mouse mast cell function. FASEB J. 1999;13:A324. [Google Scholar]
- HOGABOAM C.M., CHENSUE S.W., STEINHAUSER M.L., HUFFNAGLE G.B., LUKACS N.W., STRIETER R.M., KUNKEL S.L. Alteration of the cytokine phenotype in an experimental lung granuloma model by inhibiting nitric oxide. J. Immunol. 1997;159:5585–5593. [PubMed] [Google Scholar]
- HOGABOAM C.M., GALLINAT C.S., BONE-LARSON C., CHENSUE S.W., LUKACS N.W., STRIETER R.M., KUNKEL S.L. Collagen deposition in a non-fibrotic lung granuloma model after nitric oxide inhibition. Am. J. Pathol. 1998;153:1861–1872. doi: 10.1016/S0002-9440(10)65700-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOMEY B., ASSMANN T., VOHR H.W., ULRICH P., LAUERMA A.I., RUZICKA T., LEHMANN P., SCHUPPE H.C. Topical FK506 suppresses cytokine and costimulatory molecule expression in epidermal and local draining lymph node cells during primary skin immune responses. J. Immunol. 1998;160:5331–5340. [PubMed] [Google Scholar]
- HSIEH C.S., MACATONIA S.E., TRIPP C.S., WOLF S.F., O'GARRA A., MURPHY K.M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- HUANG F.P., NIEDBALA W., WEI X.Q., XU D., FENG G.J., ROBINSON J.H., LAM C., LIEW F.Y. Nitric oxide regulates Th1 cell development through the inhibition of IL-12 synthesis by macrophages. Eur. J. Immunol. 1998;28:4062–4070. doi: 10.1002/(SICI)1521-4141(199812)28:12<4062::AID-IMMU4062>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- JACOBSEN S.E. IL12, a direct stimulator and indirect inhibitor of haematopoiesis. Res. Immunol. 1995;146:506–514. doi: 10.1016/0923-2494(96)83024-0. [DOI] [PubMed] [Google Scholar]
- JACOBSON N.G., SZABO S.J., WEBER-NORDT R.M., ZHONG Z., SCHREIBER R.D., DARNELL J.E., JR, MURPHY K.M. Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat)3 and Stat4. J. Exp. Med. 1995;181:1755–1762. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANG K., KUBIN M., COOPER K.D., LESSIN S.R., TRINCHIERI G., ROOK A.H. IL-12 synthesis by human Langerhans cells. J. Immunol. 1996;156:1402–1407. [PubMed] [Google Scholar]
- KELLY J.J., BARNES P.J., GIEMBYCZ M.A. Characterization of phosphodiesterase 4 in guinea-pig macrophages: multiple activities, association states and sensitivity to selective inhibitors. Br. J. Pharmacol. 1998;124:129–140. doi: 10.1038/sj.bjp.0701819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KITCHING A.R., TIPPING P.G., HOLDSWORTH S.R. IL-12 directs severe renal injury, crescent formation and Th1 responses in murine glomerulonephritis. Eur. J. Immunol. 1999;29:1–10. doi: 10.1002/(SICI)1521-4141(199901)29:01<1::AID-IMMU1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- KLINMAN D.M., YI A.K., BEAUCAGE S.L., CONOVER J., KRIEG A.M. CpG motifs present in bacterial DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon γ. Proc. Natl. Acad. Sci. U.S.A. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOBAYASHI M., FITZ L., RYAN M., HEWICK R.M., CLARK S.C., CHAN S., LOUDON R., SHERMAN F., PERUSSIA B., TRINCHIERI G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEONARD J.P., WALDBURGER K.E., GOLDMAN S.J. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J. Exp. Med. 1995;181:381–386. doi: 10.1084/jem.181.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVI G., MINGHETTI L., ALOISI F. Regulation of prostanoid synthesis in microglial cells and effects of prostaglandin E2 on microglial functions. Biochimie. 1998;80:899–904. doi: 10.1016/s0300-9084(00)88886-0. [DOI] [PubMed] [Google Scholar]
- LIANG L., BESHAY E., PRUD'HOMME G.J. The phosphodiesterase inhibitors pentoxifylline and rolipram prevent diabetes in NOD mice. Diabetes. 1998;47:570–575. doi: 10.2337/diabetes.47.4.570. [DOI] [PubMed] [Google Scholar]
- LING P., GATELY M.K., GUBLER U., STERN A.S., LIN P., HOLLFELDER K., SU C., PAN Y.C., HAKIMI J. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J. Immunol. 1995;154:116–127. [PubMed] [Google Scholar]
- LOTZE M.T., ZITVOGEL L., CAMPBELL R., ROBBINS P.D., ELDER E., HALUSZCZAK C., MARTIN D., WHITESIDE T.L., STORKUS W.J., TAHARA H. Cytokine gene therapy of cancer using interleukin-12: murine and clinical trials. Ann. N.Y. Acad. Sci. 1996;795:440–454. doi: 10.1111/j.1749-6632.1996.tb52715.x. [DOI] [PubMed] [Google Scholar]
- MA X., CHOW J.M., GRI G., CARRA G., GEROSA F., WOLF S.F., DZIALO R., TRINCHIERI G. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. J. Exp. Med. 1996;183:147–157. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACATONIA S.E., HOSKEN N.A., LITTON M., VIEIRA P., HSIEH C.S., CULPEPPER J.A., WYSOCKA M., TRINCHIERI G., MURPHY K.M., O'GARRA A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J. Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- MACLEAN A., WEI X.Q., HUANG F.P., AL-ALEM U.A., CHAN W.L., LIEW F.Y. Mice lacking inducible nitric oxide synthase are more susceptible to herpes simplex virus infection despite enhanced Th1 cell responses. J. Gen. Virol. 1998;79:825–830. doi: 10.1099/0022-1317-79-4-825. [DOI] [PubMed] [Google Scholar]
- MATTNER F., OZMEN L., PODLASKI F.J., WILKINSON V.L., PRESKY D.H., GATELY M.K., ALBER G. Treatment with homodimeric interleukin-12 (IL-12) p40 protects mice from IL-12-dependent shock but not from tumor necrosis factor α-dependent shock. Infect. Immun. 1997;65:4734–4737. doi: 10.1128/iai.65.11.4734-4737.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAZZEO D., PANINA-BORDIGNON P., RECALDE H., SINIGAGLIA F., D'AMBROSIO D. Decreased IL-12 production and Th1 cell development by acetyl salicylic acid-mediated inhibition of NF-κB. Eur. J. Immunol. 1998;28:3205–3213. doi: 10.1002/(SICI)1521-4141(199810)28:10<3205::AID-IMMU3205>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- MCWHINNEY C.D., DUDLEY M.W., BOWLIN T.L., PEET N.P., SCHOOK L., BRADSHAW M., DE M., BORCHERDING D.R., EDWARDS C.K., III Activation of adenosine A3 receptors on macrophages inhibits tumor necrosis factor-α. Eur. J. Pharmacol. 1996;310:209–216. doi: 10.1016/0014-2999(96)00272-5. [DOI] [PubMed] [Google Scholar]
- METZGER D.W., VOGEL L.A., VAN CLEAVE V.H., LESTER T.L., BUCHANAN J.M. The effects of IL12 on B-cell subset function. Res. Immunol. 1995;146:499–505. doi: 10.1016/0923-2494(96)83023-9. [DOI] [PubMed] [Google Scholar]
- MOLLER D.R., WYSOCKA M., GREENLEE B.M., MA X., WAHL L., FLOCKHART D.A., TRINCHIERI G., KARP C.L. Inhibition of IL-12 production by thalidomide. J. Immunol. 1997;159:5157–5161. [PubMed] [Google Scholar]
- MORELAND L.W., BAUMGARTNER S.W., SCHIFF M.H., TINDALL E.A., FLEISCHMANN R.M., WEAVER A.L., ETTLINGER R.E., COHEN S., KOOPMAN W.J., MOHLER K., WIDMER M.B., BLOSCH C.M. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N. Engl. J. Med. 1997;337:141–147. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- MULLER G., SALOGA J., GERMANN T., BELLINGHAUSEN I., MOHAMADZADEH M., KNOP J., ENK A.H. Identification and induction of human keratinocyte-derived IL-12. J. Clin. Invest. 1994;94:1799–1805. doi: 10.1172/JCI117528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURPHY T.L., CLEVELAND M.G., KULESZA P., MAGRAM J., MURPHY K.M. Regulation of interleukin 12 p40 expression through an NF-κB half-site. Mol. Cell. Biol. 1995;15:5258–5267. doi: 10.1128/mcb.15.10.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NA S.-Y., KANG B.Y., CHUNG S.W., HAN S.-J., MA X., TRINCHIERI G., IM S.-Y., LEE J.W., KIM T.S. Retinoids inhibit interleukin-12 production in macrophages through physical association of retinoid X receptor and NFκB. J. Biol. Chem. 1999;274:7674–7680. doi: 10.1074/jbc.274.12.7674. [DOI] [PubMed] [Google Scholar]
- NÉMETH Z.H., HASKÓ G., SZABÓ C., SALZMAN A.L., VIZI E.S. Calcium channel blockers and dantrolene differentially regulate the production of interleukin-12 and interferon-γ in endotoxemic mice. Brain Res. Bull. 1998b;46:257–261. doi: 10.1016/s0361-9230(98)00005-7. [DOI] [PubMed] [Google Scholar]
- NÉMETH Z.H., HASKÓ G., VIZI E.S. Pyrrolidine dithiocarbamate augments IL-10, inhibits TNF-α, MIP-1α, IL-12, and nitric oxide production and protects from the lethal effect of endotoxin. Shock. 1998a;10:49–53. doi: 10.1097/00024382-199807000-00009. [DOI] [PubMed] [Google Scholar]
- NEURATH M.F., FUSS I., KELSALL B.L., STUBER E., STROBER W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J. Exp. Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZMEN L., PERICIN M., HAKIMI J., CHIZZONITE R.A., WYSOCKA M., TRINCHIERI G., GATELY M., GAROTTA G. Interleukin 12, interferon gamma, and tumor necrosis factor α are the key cytokines of the generalized Shwartzman reaction. J. Exp. Med. 1994;180:907–915. doi: 10.1084/jem.180.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANINA-BORDIGNON P., MAZZEO D., DI LUCIA P., D'AMBROSIA D., LANG R., FABBRI L., SELF C., SINIGAGLIA F. β2-agonists prevent TH1 development by selective inhibition of interleukin 12. J. Clin. Invest. 1997;100:1513–1519. doi: 10.1172/JCI119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J.H., SHIN S.H. Induction of IL-12 gene expression in the brain in septic shock. Biochem. Biophys. Res. Commun. 1996;224:391–396. doi: 10.1006/bbrc.1996.1038. [DOI] [PubMed] [Google Scholar]
- PASTORES S.M., HASKÓ G., VIZI E.S., KVETAN V. The role of vasoactive drugs in the regulation of cytokine production in sepsis. New Horiz. 1996;4:252–264. [PubMed] [Google Scholar]
- PETROVSKY N., MCNAIR P., HARRISON L.C. Diurnal rhythms of pro-inflammatory cytokines: regulation by plasma cortisol and therapeutic implications. Cytokine. 1998;10:307–312. doi: 10.1006/cyto.1997.0289. [DOI] [PubMed] [Google Scholar]
- PLEVY S.E., GEMBERLING J.H., HSU S., DORNER A.J., SMALE S.T. Multiple control elements mediate activation of the murine and human interleukin 12 p40 promoters: evidence of functional synergy between C/EBP and Rel proteins. Mol. Cell. Biol. 1997;17:4572–4588. doi: 10.1128/mcb.17.8.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POGGI A., RUBARTELLI A., ZOCCHI M.R. Involvement of dihydropyridine-sensitive calcium channels in human dendritic cell function. Competition by HIV-1 Tat. J. Biol. Chem. 1998;273:7205–7209. doi: 10.1074/jbc.273.13.7205. [DOI] [PubMed] [Google Scholar]
- PRESKY D.H., YANG H., MINETTI L.J., CHUA A.O., NABAVI N., WU C.Y., GATELY M.K., GUBLER U. A functional interleukin 12 receptor complex is composed of two β-type cytokine receptor subunits. Proc. Natl. Acad. Sci. U.S.A. 1996;93:14002–14007. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- RANDOW F., DÖCKE W.-D., BUNDSCHUH D.S., HARTUNG T., WENDEL A., VOLK H.-D. In vitro prevention and reversal of lipopolysaccharide desensitization by INF-γ, IL-12, and granulocyte-macrophage colony-stimulating factor. J. Immunol. 1997;158:2911–2918. [PubMed] [Google Scholar]
- REDFORD T.W., YI A.-K., WARD C.T., KRIEG A.M. Cyclosporin A enhances IL-12 production by CpG motifs in bacterial DNA and synthetic oligodeoxynucleotides. J. Immunol. 1998;161:3930–3935. [PubMed] [Google Scholar]
- REINER S.L., ZHENG S., WANG Z.E., STOWRING L., LOCKSLEY R.M. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J. Exp. Med. 1994;179:447–456. doi: 10.1084/jem.179.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIECKMANN P., WEBER F., GÜNTHER A., MARTIN S., BITSCH A., BROOCKS A., KITZE B., WEBER T., BÖRNER T., POSER S. Pentoxyfilline, a phosphodiesterase inhibitor, induces immune deviation in patients with multiple sclerosis. J. Neuroimmunol. 1996;64:193–200. doi: 10.1016/0165-5728(95)00176-x. [DOI] [PubMed] [Google Scholar]
- ROGGE L., BARBERIS-MAINO L., BIFFI M., PASSINI N., PRESKY D.H., GUBLER U., SINIGAGLIA F. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J. Exp. Med. 1997;185:825–831. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSS S.E., WILLIAMS R.O., MASON L.J., MAURI C., MARINOVA-MUTAFCHIEVA L., MALFAIT A.M., MAINI R.N., FELDMANN M. Suppression of TNF-α expression, inhibition of Th1 activity, and amelioration of collagen-induced arthritis by rolipram. J. Immunol. 1997;159:6253–6259. [PubMed] [Google Scholar]
- ROTHE H., HARTMANN B., GEERLINGS P., KOLB H. Interleukin-12 gene-expression of macrophages is regulated by nitric oxide. Biochem. Biophys. Res. Commun. 1996;224:159–163. doi: 10.1006/bbrc.1996.1000. [DOI] [PubMed] [Google Scholar]
- ROTHE H., O'HARA R.M., JR, MARTIN S., KOLB H. Suppression of cyclophosphamide induced diabetes development and pancreatic Th1 reactivity in NOD mice treated with the interleukin (IL)-12 antagonist IL-12(p40)2. Diabetologia. 1997;40:641–646. doi: 10.1007/s001250050728. [DOI] [PubMed] [Google Scholar]
- SAJJADI F.G., TABAYASHI K., FOSTER A.C., DOMINGO R.C., FIRESTEIN G.S. Inhibition of TNF-α expression by adenosine. Role of A3 adenosine receptors. J. Immunol. 1996;156:3435–3442. [PubMed] [Google Scholar]
- SHU U., KINIWA M., WU C.Y., MALISZEWSKI C., VEZZIO N., HAKIMI J., GATELY M., DELESPESSE G. Activated T cells induce interleukin-12 production by monocytes via CD40-CD40 ligand interaction. Eur. J. Immunol. 1995;25:1125–1128. doi: 10.1002/eji.1830250442. [DOI] [PubMed] [Google Scholar]
- SMITH T.J., DUCHARME L.A., WEIS J.H. Preferential expression of interleukin-12 or interleukin-4 by murine bone marrow mast cells derived in mast cell growth factor or interleukin-3. Eur. J. Immunol. 1994;24:822–826. doi: 10.1002/eji.1830240408. [DOI] [PubMed] [Google Scholar]
- SNIJDERS A., HILKENS C.M.U., VAN DER POUW KRAAN T.C.T.M., ENGEL M., AARDEN L.A., KAPSENBERG M.L. Regulation of bioactive IL-12 production in lipopolysaccharide-stimulated human monocytes is determined by the expression of the p35 subunit. J. Immunol. 1996;156:1207–1212. [PubMed] [Google Scholar]
- SOMMER N., LÖSCHMANN P.-A., NORTHOFF G.H., WELLER M., STEINBRECHER A., STEINBACH J.P., LICHTENFELS R., MEYERMANN R., RIETHMÜLLER A., FONTANA A., DICHGANS J., MARTIN R. The antidepressant rolipram suppresses cytokine production and prevents autoimmune encephalomyelitis. Nat. Medicine. 1995;1:244–248. doi: 10.1038/nm0395-244. [DOI] [PubMed] [Google Scholar]
- SOUNESS J.E., GRIFFIN M., MASLEN C., EBSWORTH K., SCOTT L.C., POLLOCK K., PALFREYMAN M.N., KARLSSON J.A. Evidence that cyclic AMP phosphodiesterase inhibitors suppress TNF-α generation from human monocytes by interacting with a ‘low-affinity' phosphodiesterase 4 conformer. Br. J. Pharmacol. 1996;118:649–658. doi: 10.1111/j.1476-5381.1996.tb15450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUSA C.R., HIENY S., SCHARTON-KERSTEN T., JANKOVIC D., CHAREST H., GERMAIN R.N., SHER A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STALDER A.K., PAGENSTECHER A., YU N.C., KINCAID C., CHIANG C.S., HOBBS M.V., BLOOM F.E., CAMPBELL I.L. Lipopolysaccharide-induced IL-12 expression in the central nervous system and cultured astrocytes and microglia. J. Immunol. 1997;159:1344–1351. [PubMed] [Google Scholar]
- SZABÓ C., HASKÓ G., NÉMETH Z.H., VIZI E.S. Calcium entry blockers increase interleukin-10 production in endotoxemia. Shock. 1997a;7:304–307. doi: 10.1097/00024382-199704000-00011. [DOI] [PubMed] [Google Scholar]
- SZABÓ C., SCOTT G.S., VIRÁG L., EGNACZYK G., SALZMAN A.L., SHANLEY T.P., HASKÓ G. Suppression of macrophage inflammatory protein (MIP)-1α production and collagen-induced arthritis by adenosine receptor agonists. Br. J. Pharmacol. 1998;125:379–387. doi: 10.1038/sj.bjp.0702040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABO S.J., DIGHE A.S., GUBLER U., MURPHY K.M. Regulation of the interleukin (IL)-12R β2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J. Exp. Med. 1997b;185:817–824b. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI M., OGASAWARA K., TAKEDA K., HASHIMOTO W., SAKIHARA H., KUMAGAI K., ANZAI R., SATOH M., SEKI S. LPS induces NK1.1+ alpha beta T cells with potent cytotoxicity in the liver of mice via production of IL-12 from Kupffer cells. J. Immunol. 1996;156:2436–2442. [PubMed] [Google Scholar]
- TAKANO M., NISHIMURA H., KIMURA Y., WASHIZU J., MOKUNO Y., NIMURA Y., YOSHIKAI Y. Prostaglandin E2 protects against liver injury after Escherichia coli infection but hampers the resolution of the infection in mice. J. Immunol. 1998;161:3019–3025. [PubMed] [Google Scholar]
- TAOUFIK Y., LANTZ O., WALLON C., CHARLES A., DUSSAIX E., DELFRAISSY J.F. Human immunodeficiency virus gp120 inhibits interleukin-12 secretion by human monocytes: an indirect interleukin-10-mediated effect. Blood. 1997;89:2842–2848. [PubMed] [Google Scholar]
- TREMBLEAU S., PENNA G., BOSI E., MORTARA A., GATELY M.K., ADORINI L. Interleukin 12 administration induces T helper type 1 cells and accelerates autoimmune diabetes in NOD mice. J. Exp. Med. 1995;181:817–821. doi: 10.1084/jem.181.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREPICCHIO W.L., BOZZA M., PEDNEAULT G., DORNER A.J. Recombinant human IL-11 attenuates the inflammatory response through down-regulation of proinflammatory cytokine release and nitric oxide production. J. Immunol. 1996;157:3627–3634. [PubMed] [Google Scholar]
- TRINCHIERI G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- TRIPP C.S., WOLF S.F., UNANUE E.R. Interleukin 12 and tumor necrosis factor α are costimulators of interferon γ production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc. Natl. Acad. Sci. U.S.A. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UYEMURA K., DEMER L.L., CASTLE S.C., JULLIEN D., BERLINER J.A., GATELY M.K., WARRIER R.R., PHAM N., FOGELMAN A.M., MODLIN R.L. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J. Clin. Invest. 1996;97:2130–2138. doi: 10.1172/JCI118650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER POUW KRAAN T.C.T.M., BOEIJE L.C.M., SMEENK R.J.T., WIJDENES J., AARDEN L.A. Prostaglandin-E2 is a potent inhibitor of human interleukin-12 production. J. Exp. Med. 1995;181:775–779. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER POUW KRAAN T.C., SNIJDERS A., BOEIJE L.C., DE GROOT E.R., ALEWIJNSE A.E., LEURS R., AARDEN L.A. Histamine inhibits the production of interleukin-12 through interaction with H2 receptors. J. Clin. Invest. 1998;102:1866–1873. doi: 10.1172/JCI3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERGHESE M.W., MCCONNELL R.T., STRICKLAND A.B., GOODING R.C., STIMPSON S.A., YARNALL D.P., TAYLO J.D., FURDON P.J. Differential regulation of human monocyte-derived TNF-α and IL-1β by type IV cAMP-phosphodiesterase (cAMP-PDE) inhibitors. J. Pharmacol. Exp. Ther. 1995;272:1313–1320. [PubMed] [Google Scholar]
- VIEIRA P.L., KALINSKI P., WIERENGA E.A., KAPSENBERG M.L., DE JONG E.C. Glucocorticoids inhibit bioactive IL-12p70 production by in vitro-generated human dendritic cells without affecting their T cell stimulatory potential. J. Immunol. 1998;161:5245–5251. [PubMed] [Google Scholar]
- WILCKENS T., DE RIJK R. Glucocorticoids and immune function: unknown dimensions and new frontiers. Immunol. Today. 1997;18:418–424. doi: 10.1016/s0167-5699(97)01111-0. [DOI] [PubMed] [Google Scholar]
- WU C.-Y., WANG K., MCDYER J.F., SEDER R.A. Prostaglandin E2 and dexamethasone inhibit IL-12 receptor expression and IL-12 responsiveness. J. Immunol. 1998;161:2723–2730. [PubMed] [Google Scholar]
- WU C., WARRIER R.R., WANG X., PRESKY D.H., GATELY M.K. Regulation of interleukin-12 receptor β1 chain expression and interleukin-12 binding by human peripheral blood mononuclear cells. Eur. J. Immunol. 1997;27:147–154. doi: 10.1002/eji.1830270122. [DOI] [PubMed] [Google Scholar]
- WYSOCKA M., KUBIN M., VIEIRA L.Q., OZMEN L., GARROTTA G., SCOTT P., TRINCHIERI G. Interleukin-12 is required for interferon-γ production and lethality in lipopolysaccharide-induced shock in mice. Eur. J. Immunol. 1995;25:672–676. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]
- XIN Z., SRIRAM S. Vasoactive intestinal peptide inhibits IL-12 and nitric oxide production in murine macrophages. J. Neuroimmunol. 1998;89:206–212. doi: 10.1016/s0165-5728(98)00140-4. [DOI] [PubMed] [Google Scholar]
- YAWALKAR N., KARLEN S., HUNGER R., BRAND C.U., BRAATHEN L.R. Expression of interleukin-12 is increased in psoriatic skin. J. Invest. Dermatol. 1998;111:1053–1057. doi: 10.1046/j.1523-1747.1998.00446.x. [DOI] [PubMed] [Google Scholar]
- YE J., ORTALDO J.R., CONLON K., WINKLER-PICKETT R., YOUNG H.A. Cellular and molecular mechanisms of IFN-γ production induced by IL-2 and IL-12 in a human NK cell line. J. Leuk. Biol. 1995;58:225–233. doi: 10.1002/jlb.58.2.225. [DOI] [PubMed] [Google Scholar]
- ZHANG M., GATELY M.K., WANG E., GONG J., WOLF S.F., LU S., MODLIN R.L., BARNES P.F. Interleukin 12 at the site of disease in tuberculosis. J. Clin. Invest. 1994;93:1733–1739. doi: 10.1172/JCI117157. [DOI] [PMC free article] [PubMed] [Google Scholar]


