Abstract
Subtypes of α1-adrenoceptor in rabbit iris have been examined in functional, binding and molecular biological experiments.
In functional studies, exogenous and endogenous noradrenaline produced contractions of the iris dilator muscle. The contractile responses to noradrenaline were competitively antagonized by a range of α1-adrenoceptor antagonists (pA2 values): prazosin (8.1), WB4101 (8.2), BMY7378 (5.9), YM617 (9.5), JTH-601 (8.8), HV723 (7.8) and KMD-3213 (9.8). The same order of inhibitory potency was seen in the adrenergic responses to electrical stimulation. This affinity profile corresponds well to that of the putative α1L-adrenoceptor, which has been proposed in lower urinary tract tissues.
In binding studies on rabbit iris membrane however, prazosin, KMD-3213 and WB4101 displayed high affinity (pKd or pKi: 9.6, 10.3, 9.6, respectively), and BMY7378 displayed low affinity (pKi: 6.9). These results show that the binding sites typically correspond to α1A-adrenoceptor subtype in character, and we could not detect the significant amount of α1L-adrenoceptor subtype.
The expression of the three distinct mRNAs that encode proteins of α1a-, α1b- and α1d-adrenoceptors was studied using reverse transcription-polymerase chain reaction (RT–PCR). RT–PCR demonstrated the strongest expression of the α1a-adrenoceptor, weak expression of the α1b- adrenoceptor and undetectable expression of the α1d-adrenoceptor.
The present study suggests that α1A-adrenoceptor is a major subtype detectable in binding and RT–PCR studies in rabbit iris, but that the adrenergic contractions of iris dilator muscle are mediated via activation of α1-adrenoceptor subtype having low affinity for prazosin and WB4101.
Keywords: Rabbit, iris, dilator muscle, α1-adrenoceptor, subtype, radioligand, mRNA
Introduction
Pharmacological and molecular cloning studies have established operational and structural heterogeneity among α1-adrenoceptors (Minneman, 1988; Ford et al., 1994; Bylund et al., 1994). The current classification recognizes the existence of three α1-adrenoceptors (α1A, α1B and α1D) all of which meet well the criteria (i.e., sequence, second messenger and pharmacological profile: Hieble et al., 1995). A fourth α1-adrenoceptor, α1L-adrenoceptor, which is mainly based on the results of functional pharmacological studies, has been postulated (Holck et al., 1983; Flavahan & Vanhoutte, 1986; Muramatsu, 1992). However, the corresponding gene has not been identified yet. Being widely distributed in heart, liver, lower urinary tract tissues, vascular and ocular smooth muscle tissues, α1-adrenoceptors play an important physiological role in peripheral organs (Lefkowitz & Caron, 1988; Minneman, 1988; Muramatsu, 1992; Ford et al., 1996; Miyamoto et al., 1997; Suzuki et al., 1997).
The iris, which controls the size of the pupil and thus regulates the amount of light admitted, is innervated by sympathetic and parasympathetic nerves; the former regulates the contraction of dilator muscle via α1-adrenoceptors (Malmfors, 1965; Van Alphen et al., 1965; Narita & Watanabe, 1982; Konno & Takayanagi, 1986; McGregor, 1994) and the latter controls that of sphincter muscle via muscarinic receptors (Choppin et al., 1998) in many mammalian species. Thus, agonism of α1-adrenoceptors in the iris dilator leads to mydriasis, conversely antagonism results in miosis. Ishikawa et al., (1996) reported that the α1-adrenoceptor subtype in rabbit iris dilator has high affinity for prazosin, while that in the iris dilator of human or pigmented rabbit has low affinity for prazosin. Mittag & Tormay (1985) reported that the pKd value of [3H]-prazosin is 9.3 in rabbit iris-ciliary body. However, because of a small amount of this tissue, very few binding studies have been made in the iris so far, and it still remains unclarified which subtype(s) of α1-adrenoceptors are involved in the contraction of the dilator muscle.
The purpose of this study is to demonstrate the α1-adrenoceptor subtype responsible for the rabbit iris dilator contraction in the functional study, and to characterize the α1-adrenoceptor subtype of whole rabbit iris in the binding study and RT–PCR assay.
Methods
Animals
Tissues were obtained from adult male albino rabbits that weighed 2–2.5 kg. Rabbits were anaesthetized with intravenous sodium pentobarbital (50 mg kg−1) and killed. The eyes were enucleated promptly and dissected under a surgical microscope to isolate iris in ice-cold modified Krebs-Henseleit solution of the following composition (mM): NaCl 112, KCl 5.9, MgCl2 1.2, CaCl2 2, NaHCO3 25, NaH2PO4 1.2, and glucose 11.5.
Functional experiments
A strip of iris dilator muscle was prepared in approximately 2 mm width and 4 mm length and was mounted vertically in an organ bath containing 20 ml of modified Krebs-Henseleit solution, continuously bubbled with 95% O2 and 5% CO2 at 37°C. All preparations were equilibrated for 90 min with a resting tension of 50 mg before the experiments were begun. Changes in isometric tension were recorded on a pen-writing oscillograph (FJG-4128, Nihon Koden, Tokyo, Japan) in the following experiments.
Concentration-response curves for agonists were obtained by adding the drugs directly to the bath in cumulative fashion. The concentration-response curves for noradrenaline were obtained four times in the same strip at 60 min washout intervals. The second concentration-response curves were used as the control and α1-adrenoceptor antagonists were added for 30 min before and during the third and fourth responses. In all experiments desipramine (0.1 μM), deoxycorticosterone acetate (1 μM) and propranolol (1 μM) were added in the bath to block neural and extraneural uptake of noradrenaline and to block β-adrenoceptors, respectively.
For the transmural electrical stimulation (TES), the preparation was placed between a pair of platinum electrodes. Gaps between the electrodes were wide enough to accommodate the strip and yet sufficiently narrow to stimulate the intramural nerve terminals effectively. To block presynaptic α2-adrenoceptor, DG-5128 (10 μM) was added to the bath (Muramatsu et al., 1983). Although Yamanaka et al. (1984) showed that IC50 value of this compound at α1-adrenoceptors of rat brain homogenates is approximately 4 μM, we confirmed that noradrenaline-induced contractions of the rabbit dilator muscle are not affected by DG-5128 up to 10 μM in preliminary examination (data not shown). A train of rectangular pulses (7.5 mV intensity, 130 μs duration, 10 Hz frequency, for 10 s) was applied every 15 min. Antagonists to be tested were added to the organ chamber 30 min before the stimulation.
The affinities for α1-adrenoceptor antagonists were estimated according to Arunlakshana & Schild (1959). Data were plotted as the −log [antagonist concentration] vs the log [concentration ratio (CR) −1], and pA2 values with mean slopes and 95% confidence limits (95% CL) were calculated and first regression lines were drawn by least square method.
Binding experiments
For membrane preparation the irides were minced with scissors and homogenized in 20 volume of buffer (Tris HCl 50 mM, NaCl 100 mM, EDTA 2 mM, pH 7.4) with a polytron homogenizer (setting 8, 15 s×3) and centrifuged at 3000×g for 15 min. The supernatant was filtered through four layers of surgical gauze and subjected to centrifugation at 80,000×g for 30 min. The pellet was resuspended in ice-cold assay buffer (Tris HCl 50 mM, EDTA 1 mM, pH 7.4) and the resuspension was centrifuged at 80,000×g for 30 min again. All centrifugation was done at 4°C. The resultant pellet was resuspended in assay buffer as a membrane fraction and was immediately used for binding assay.
In saturation binding experiments, the membranes were incubated with various concentrations of [3H]-ligand ([3H]-prazosin; 30 pM–5 nM or [3H]-KMD; 10 pM–1 nM) for 45 min at 30°C with incubation volume 1 and 2 ml, respectively. Nonspecific binding was defined as binding in the presence of a masking ligand (0.3 μM YM617 for [3H]-prazosin and 0.3 μM prazosin for [3H]-KMD). In competition binding experiments, the membranes were incubated with about 100 pM [3H]-KMD and unlabelled drugs for 45 min at 30°C. Reactions were terminated by rapid filtration through Whatman GF/C filters presoaked in 0.3% polyethylenimine for 45 min. The filters were then washed four times with 4 ml of ice-cold 50 mM Tris-HCl buffer (pH 7.4) and dried. The filter-bound radioactivity was determined by liquid scintillation counting. All assays were performed in duplicate. Binding affinities of [3H]-ligands and unlabelled drugs were expressed as negative logarithm of the equilibrium dissociation constant (pKd and pKi, respectively). Displacement binding data were analysed by GraphPAD Prism. When the slope factor was close to unity, concentrations of competing agent producing 50% displacement of [3H]-KMD (IC50) were converted to Ki values with the Cheng & Prusoff approximation (1973). Protein determination was assayed according to the method of Bradford (1976) with bovine serum albumin as standard.
RT–PCR assays
The iris was frozen in liquid nitrogen immediately after isolation and then stored at −80°C until extraction of RNA. Total cellular RNA was extracted according to the procedure of Chomczynski & Sacchi (1987). All RNA transcripts for three adrenoceptor subtypes were synthesized from the appropriate plasmid DNA with the use of T7 RNA polymerase (Gibco BRL). To produce the cDNA, total RNA (500 ng) from iris and cRNA (300 pg) from clones were individually reverse transcribed by moloney murine leukaemia virus reverse transcriptase (Gibco BRL) with random hexamer. Three μl of cDNA was amplified in a 25 μl PCR mixture containing (mM) KCl 50, Tris-HCl 10, pH 8.3, MgCl2 2, 200 μM each of dNTP, 0.25 μM sense and antisense primers and 2.5 U Pwo DNA polymerase (Boehringer). The subtype specific primers were designed from published sequences of rabbit α1a- and α1d-adrenoceptors (Miyamoto et al., 1997; Suzuki et al., 1997) and unpublished sequence of rabbit α1b-adrenoceptor, as follows: α1a: 5′-CATCGTGGTCGGCTGCTTCGTC-3′, 5′-GGCTGTAGTGCAGGCTGATT-3′, α1b: 5′-AGGAGCCGGCACCCAATGATGA-3′, 5′-GGCACTGGCACCCGAGGAT-3′, α1d: 5′-CTCCGTGCGCCTGCTCAAGT-3′, 5′-GGGTAGATGAGTGGGTTCAC-3′. PCR was performed in a Perkin-Elmer DNA Thermal Cycler 2400 with the following cycle parameters: one denaturation cycle for 3 min at 98°C; 40 cycles of 30 s at 95°C, annealing for 10 s and extension for 15 s at 72°C. Annealing temperatures were 68°C for the α1a-adrenoceptor, 64°C for the α1b-and α1d-adrenoceptor. For each experiment, negative controls were amplified, in which all of the components except reverse transcriptase were included. The PCR products were separated on 3.5% polyacrylamide gels and stained with ethidium bromide.
Drugs
The drugs used and their sources were following: (−)-(R)-1-(3-hydroxypropyl)-5-[2-[[2-[2-(2,2,2-trifluoroethoxy) phenoxy] ethyl]amino]propyl]indoline-7-carboxamide (KMD-3213) and tamsulosin hydrochloride (YM-617) from Kissei Pharmaceutical Co. Ltd. (Matsumoto, Japan); 3-[N-[2-[4-hydroxy-2-isopropyl-5-methylphenoxy] ethyl] -N-methylaminomethyl] - 4-methoxy-2,5,6-trimethylphenol hemifumarate (JTH-601) from Japan Tobacco Inc. (Osaka, Japan); α-ethyl-3,4,5-trimethoxy-α-[3-[ [2-2-methoxyphenoxy]ethyl] -amino] -propyl] benzeneacetonitrile fumarate] (HV723) from Hokuriku Seiyaku (Katsuyama, Japan); [2-[2-[4,5-dihydro-1H-imidazol-2-yl]1-phenylethyl] pyridine dihydrochloride sesquihydrate] (DG-5128) from Daiichi Seiyaku (Tokyo, Japan); prazosin hydrochloride, (−)-noradrenaline bitartrate, (−)-phenylephrine hydrochloride, oxymetazoline and desipramine hydrochloride, from Sigma (St. Louis, U.S.A.); WB4101 and BMY7378 from Research Biochemicals Inc. (Natick, U.S.A.); (−)-propranolol hydrochloride and deoxycorticosterone acetate from Nakalai (Kyoto, Japan); clonidine hydrochloride from Funakoshi (Tokyo, Japan); [3H]-KMD-3213 (49 Ci mmol−1), from Amersham (England); and [3H]-prazosin (77.2 Ci mmol−1) from NEN (Boston, U.S.A.).
Results
Functional study
Figure 1 shows the concentration-response curves for noradrenaline, phenylephrine, oxymetazoline and clonidine in the rabbit iris dilator muscle. All agonists produced concentration-dependent contraction in dilator muscle. The maximal amplitude of contraction induced by phenylephrine was almost equal to that induced by noradrenaline, whereas those by oxymetazoline or clonidine were smaller than that induced by noradrenaline. The pEC50 values and relative contractions of these agonists are summarized in Table 1, in which the maximal amplitude of contraction was compared with that induced by noradrenaline in the same strip. The following order of potency was observed: oxymetazoline > noradrenaline > phenylephrine > clonidine. Several α1-adrenoceptor antagonists (prazosin, WB4101, BMY7378, YM617, JTH-601, HV723 and KMD-3213) were tested for their ability to inhibit noradrenaline-induced responses and their functional affinity estimates (pA2 values) are summarized in Table 2. The concentration-response curves for noradrenaline were antagonized by these compounds in a concentration-dependent manner, with parallel rightward displacements. Figure 2 shows representative results with prazosin and KMD-3213. The Schild slopes were not significantly different from unity for all these antagonists, indicating that the antagonism was competitive in nature. KMD-3213, YM617 and JTH-601 displayed higher affinity than the other antagonists (Table 2). Rauwolscine (1 μM) had no effect on noradrenaline-induced contraction (data not shown).
Figure 1.
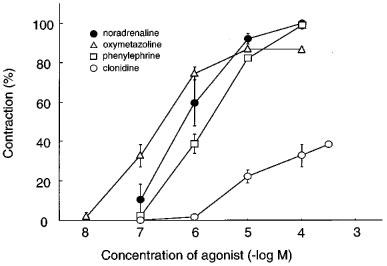
Concentration-response curves for four α-adrenoceptor agonists in isolated rabbit iris dilator muscle. The maximal contraction induced by 100 μM noradrenaline (267.2±19.4 mN; n=8) was taken as 100% in each preparation. The data shown are means±s.e.mean of 4–8 experiments. For pEC50 values and maximal contractions see Table 1.
Table 1.
Contractile parameters for various α-adrenoceptor agonists in isolated rabbit iris dilator
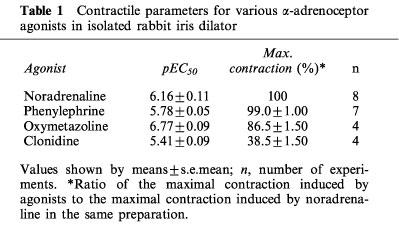
Table 2.
Functional affinities for α1-adrenoceptor antagonists in isolated rabbit iris dilator
Figure 2.
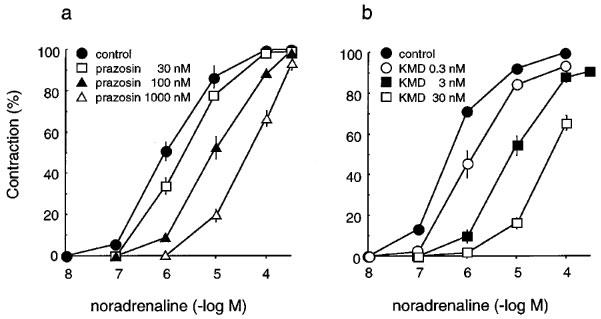
Concentration-response curves of isolated rabbit iris dilator muscle for noradrenaline and effects of prazosin (a) and KMD-3213 (b). Responses were plotted as percentages of maximal contractile response for noradrenaline in control. The data shown are means±s.e.mean of 3–6 experiments. For pA2 values see Table 2.
TES at 10 Hz for 10 s produced a monophasic and transient contraction of dilator muscle. The responses to TES were abolished by 0.5 μM tetrodotoxin. The α1-adrenoceptor antagonists mentioned above inhibited but not abolished the contraction induced by TES; thus 15% of contractile amplitude remained as the resistant component (Muramatsu et al., 1994). Their functional affinities (pIC50 which was estimated at the level of 50% reduction of α1-adrenoceptor antagonist-sensitive contraction) are also summarized in Table 2. The rank order of pIC50 was the same as pA2 values estimated in noradrenaline-response.
Binding study
Figure 3 shows representative data obtained in saturation experiments with [3H]-prazosin (a) and [3H]-KMD (b), respectively. Both [3H]-prazosin and [3H]-KMD meet the criteria for binding to a single set of sites. The binding isotherms for these ligands were hyperbolic, as shown in Figure 3. The pKd values and maximum binding sites (Bmax) of both ligands are shown in Table 3. Although we could not carry out many experiments because of limitation of tissue amount, there was no difference between the two independent experiments (Experiment 1 and Experiment 2). The pKd values of [3H]-prazosin and [3H]-KMD are 9.6 and 10.3 (mean of two experiments), respectively, and Bmax values of [3H]-prazosin and [3H]-KMD were almost same (Table 3). The specific bindings of [3H]-KMD were displaced by simultaneous addition of nonradioactive ligands (Figure 4). The slope factors of the drugs were not significantly different from unity, suggesting the presence of a single type of binding site. The pKi values for drugs in two independent experiments were almost same (Table 3).
Figure 3.
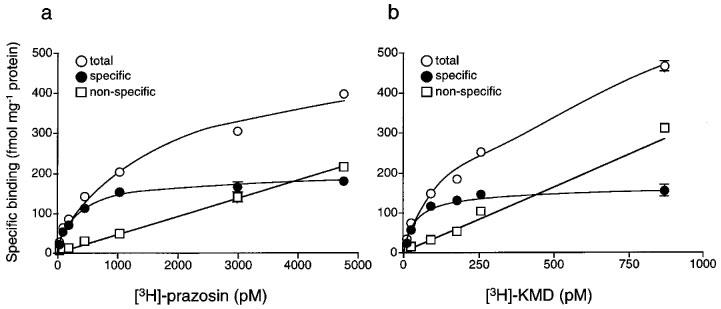
Saturation experiments of [3H]-prazosin (a) and [3H]-KMD (b) binding to rabbit iris membranes. The data are obtained from a single experiment (Experiment 1) where each point is the mean of duplicate determinations. Another experiment was also carried out with different membrane preparation and the data obtained from two independent experiments were shown in Table 3.
Table 3.
Binding parameters estimated with [3H]-prazosin and [3H]-KMD in rabbit iris membranes
Figure 4.
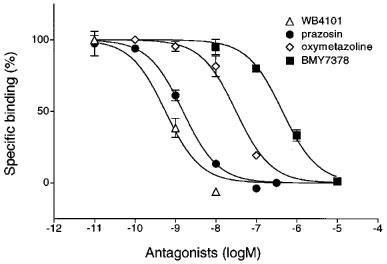
Displacement of [3H]-KMD binding to rabbit iris membranes by α1-adrenoceptor antagonists. The concentration of [3H]-KMD used was 100 pM. The data are obtained from a single experiment (Experiment 2) where each point is the mean of duplicate determinations. The data obtained from two independent experiments were shown in Table 3.
RT–PCR study
Figure 5 illustrates the results of a representative experiment using total RNA from rabbit iris and cRNAs from cloned α1-adrenoceptor subtypes. The RT–PCR products derived from clone cRNAs are observed for α1a-adrenoceptor at 442 bp (A, lane 4), α1b-adrenoceptor at 549 bp (B, lane 4) and α1d-adrenoceptor at 210 bp (C, lane 4), respectively. The cDNA generated from total RNA of irides was amplified and expression of α1-adrenoceptor subtypes mRNA in rabbit iris is shown in lanes 2. The α1a-adrenoceptor specific oligonucleotide primers amplified a large amount of cDNA (A, lane 2). A faint band was generated with the α1b-adrenoceptor specific primers (B, lane 2) while no detectable PCR product was amplified with the α1d-adrenoceptor specific primers (C, lane 2). All PCR fragments corresponded to the predicted fragment size for each receptor subtype. No PCR product was seen in the control experiments which did not contain reverse transcriptase in the reverse transcriptase-reaction (A,B,C, lanes 3 and 5), suggesting no DNA contamination was present. Two other experiments conducted under same conditions showed the identical results.
Figure 5.
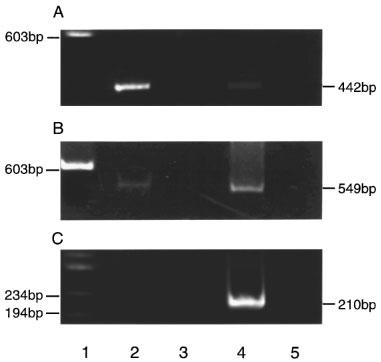
Polyacrylamide gel electrophoresis of 5 μl of RT–PCR product. Lane 1 shows DNA size markers ΦX174/HaeIII. Lanes 2 and 3 represent adrenoceptor RT–PCR products from iris total RNA. Lanes 4 and 5 represent α1a-, α1b-, and α1d-adrenoceptor RT–PCR products from 300 pg of each adrenoceptor clone cRNA. Bands of lane 4 442 bp (A), 549 bp (B), 210 bp (C) is a product of the α1a-, α1b- and α1d-adrenoceptor clone cRNA respectively. No PCR products were seen in the control experiments, which contained no reverse transcriptase in the reverse transcriptase-reaction (lanes 3 and 5).
Discussion
In the functional study, a series of α-adrenoceptor agonists and antagonists, which should be able to discriminate the subtype involved (Hieble et al., 1995; Muramatsu et al., 1998), were used to characterize the α-adrenoceptors in the dilator smooth muscle of rabbit iris. Noradrenaline and phenylephrine acted as full agonists, while oxymetazoline and clonidine behaved as partial agonists (Figure 1 and Table 1). The contractile responses to noradrenaline and TES were inhibited in a concentration-dependent and competitive fashion by several α1-adrenoceptor selective antagonists (Figure 2). Whereas the responses to noradrenaline were not inhibited by 1 μM of rauwolscine (a selective antagonist for α2-adrenoceptor). These results indicate that the adrenergic responses in rabbit iris dilator are mediated through α1-adrenoceptors. However, the potencies or affinities varied among the agonists or antagonists used (Tables 1 and 2), suggesting an involvement of unique α1-adrenoceptors in the adrenergic responses. Since Schild slopes for tested antagonists were close to unity and the range of effective concentrations of tested agonists were less than three log units, it appears that the adrenergic contractions are predominantly mediated by a single α1-adrenoceptor subtype. In the series of antagonists tested, KMD-3213 (pA2: 9.8), YM617 (9.5) and JTH-601 (8.8) showed high affinity, while WB4101 (8.2), prazosin (8.1), HV723 (7.8) and BMY7378 (5.9) showed low affinity. These affinity estimates of antagonists correlated most closely with those in lower urinary tract tissues of rabbit or human (pA2: prazosin 7.85–8.26; WB4101 8.4–8.9; BMY7378 6.4; YM617 9.31–9.49; JTH-601 8.8; HV723 7.63–8.05; KMD-3213 9.9–10.05) (Honda et al., 1985; Ford et al., 1996; Yamagishi et al., 1996; Leonardi et al., 1997; Takahashi et al., 1998; Murata et al., 1999), where putative α1L-adrenoceptor has been proposed in the adrenergic responses. Furthermore, the affinity values for these antagonists against the responses to TES showed the same trend (Table 2). These results suggest that exogenous and endogenous noradrenaline acts on the same subtype (presumably α1L-adrenoceptor) to cause contraction in rabbit iris dilator.
The α1L-adrenoceptor subtype is characterized according to low affinity for prazosin and other antagonists (Flavahan & Vanhoutte, 1986; Muramatsu et al., 1990). Marshall et al. (1996) argued that one of the main problems in determining whether prazosin does distinguish between α1-adrenoceptor subtypes is that the pA2 values published cover a continuous range of values rather than falling into discrete groups. For example, pA2 values of 8.3, 8.2 and 8.1 have been documented for α1-adrenoceptors in the rabbit trigone, urethra and prostate, respectively (Honda et al., 1985), 8.5 in rabbit prostate (Williams & Clarke, 1993), 8.9 in rat anococcygeus (Ford et al., 1993) and rat portal vein (Schwietert et al., 1991), 9.2 in rat epididymal vas deferens, spleen and portal vein (Burt et al., 1995), 9.3, 9.5, and 9.6 in rat perfused kidney, perfused mesentery and aortic rings respectively (Ford et al., 1996) and 9.8 in rat aorta (Kenny et al., 1995). In this study, however, the pA2 value of 8.1 obtained for prazosin on the rabbit iris dilator is low enough to show the involvement of α1L-adrenoceptors in the contractile response of iris dilator muscle.
In the binding study, however, not only [3H]-KMD but also [3H]-prazosin bound to the iris membranes with subnanomolar high affinity (pKd: 10.3 for [3H]-KMD and 9.6 for [3H]-prazosin, respectively) and with the same density (Figure 3 and Table 3). Furthermore, [3H]-KMD binding sites were recognized as prazosin- and WB4101- high affinity sites (Figure 4 and Table 3). Originally, KMD-3213 was reported as an α1A-adrenoceptor-selective antagonist (Shibata et al., 1995). In the experiments with human recombinant adrenoceptors, KMD-3213 showed a pKi value of 10.4 for the α1a-adrenoceptor, but has 580- and 56 fold lower affinity for α1b and α1d-adrenoceptors, respectively. Recently, it was also reported that KMD-3213 shows high affinity not only for α1a- but also for α1L-adrenoceptor (pA2: 9.9) (Murata et al., 1999). This high selectivity of KMD-3213 to α1L-adrenoceptor was confirmed in the present functional studies (Table 2). So it is likely that Bmax of [3H]-KMD consist of major α1A and minor α1L site, whereas considering the result from RT–PCR studies, Bmax of [3H]-prazosin consist of major α1A and minor α1B site. Although we could not carry out further analysis because of limitation of tissue amount, the results obtained clearly show that the binding sites correspond mainly to α1A-adrenoceptor subtype in character, and that the significant amount of α1L-adrenoceptor subtype is undetectable.
The results on mRNA expression of α1-adrenoceptor subtypes agrees with the result of binding experiments. RT–PCR studies demonstrated that expression of mRNA for the α1a-adrenoceptor is major and that those of the α1b- and α1d-adrenoceptors are minor or negligible, respectively. Although mRNA levels do not always correlate with protein expression, the predominance of mRNA for α1a-adrenoceptor suggests that this receptor may play an important role in irideal function.
Thus, there is a discrepancy for α1-adrenoceptor subtypes between functional and binding or RT–PCR studies. How do we consider this matter? First of all, we must consider experimental conditions. Williams et al. (1996) demonstrated that in binding studies of the human cloned α1A-adrenoceptor, temperature and cellular integrity appear to influence affinity estimates for several key antagonists, resulting in an apparent profile as α1L-adrenoceptor. We tested various conditions including those employed in this study, however, rabbit cloned α1A-adrenoceptor expressed in Chinese hamster ovary (CHO) cells displayed the binding affinity profile as α1A-adrenoceptor (data not shown).
Second, characteristics of compounds such as high lipophilicity, chemical instability and extra binding sites, are likely to underestimate the affinity constant. For example, a low affinity estimate of S-niguldipine in the human lower urinary tract is probably influenced by its high lipophilicity (Ford et al., 1996). For another example, the human α1-acid glycoprotein can inhibit prazosin and WB4101 binding to α1A-adrenoceptors and therefore reduce their affinity for these receptors (Chiang et al., 1991; Qin & Øie, 1994). However, because the Schild regressions performed for all antagonists used in our experiments validated that the inhibition curve design and the slopes were close to unity, these influences may be negligible.
Third, in most binding studies, the concentrations of [3H]-prazosin were up to 1 nM and then a single high affinity constant for prazosin (pKd close to 10) has been estimated. However, it should be noted that affinity constant (KB) of the α1L-adrenoceptors found in the functional studies is close to 10 nM. Thus, it is likely that α1L-adrenoceptors will be overlooked when low concentrations (<1 nM) of the [3H]-prazosin are used. Although in this study we used up to 5 nM of [3H]-prazosin, revealing the apparently saturated binding to α1-adrenoceptors of rabbit iris (Figure 3a), this concentration might not be enough to detect α1L-adrenoceptor subtype.
Fourth, it is likely that α1A-adrenoceptor is a predominant subtype while α1L-adrenoceptor is a minor in whole rabbit iris. The iris consists of four layers; anterior border layer, stroma and sphincter muscle layer, anterior epithelium and dilator muscle layer, posterior pigmented epithelium layer. In these, the dilator muscle is a very minor element (in human; 60 μm long and 7 μm wide) whereas the major structural element is the stroma which contains the vessels, nerves and cellular compartments (Bron et al., 1997). Previous reports showed that prazosin and bunazosin lower intraocular pressure in rabbits by a reduction in the rate of aqueous humor formation, which could occur by a vascular mechanism without affecting the pupillary diameter (Krupin et al., 1980; Green & Hatchett, 1987; Nishimura et al., 1993; Hong et al., 1998). These results suggest that there are at least two sets of α1-adrenoceptors; one is high sensitive to prazosin in blood vessels and another shows low affinity to prazosin located in iris dilator muscle. Thus, it may be possible to speculate that α1A-adrenoceptor distributes in the stroma, probably in the vessels, and shares the major part of α1-adrenoceptor in whole iris while α1L-adrenoceptor locates in the dilator muscle, as suggested in this study, and shares the small part.
Finally, a possible explanation for the discrepancy between functional and binding or molecular experiments is based on findings that functional estimates of antagonist affinity for the α1a-adrenoceptor correlate closely with those established for the α1L-adrenoceptor (Ford et al., 1997). Ford et al. compared binding affinities estimated by displacement of [3H]-prazosin in membrane homogenates of CHO cells stably expressing the human α1a-, α1b-, and α1d-adrenoceptors with affinity estimates obtained functionally in identical cells by measuring inhibition of noradrenaline-stimulated accumulation of [3H]-inositol phosphates. And they suggested that upon functional pharmacological analysis, the cloned α1a-adrenoceptor displays pharmacological recognition properties consistent with those of the putative α1L-adrenoceptor. Recently, four isoforms of the α1a-adrenoceptor generated by alternative splicing, which differ in length and sequence at the carboxy-terminal region, have been identified in human (Hirasawa et al., 1995; Chang et al., 1998). The α1a-adrenoceptor of rabbit also have at least three splice variants (unpublished result), however, the physiological significance of α1a-adrenoceptor splice variants is presently unknown. Since the isoforms differ only in their carboxy-terminal regions, they may differ in their preference for G-protein coupling or in rates of desensitization mediated by phosphorylation. Recent data have implicated that some splice variants may demonstrate α1L-adrenoceptor pharmacology in functional studies (Chang et al., 1998). However, why functional profile differs from that obtained in binding study requires further explanation.
In conclusion, we have found that α1A-adrenoceptor is a major subtype in the α1-adrenoceptors distributing in rabbit iris from binding study and that mRNA encoding this subtype is predominant in whole rabbit iris. While the dilator muscle, one of the constituents of the iris contracts via activation of α1-adrenoceptor having low affinity for prazosin, WB4101 and BMY7378. This discrepancy is highly debatable, including the controversy whether or not the gene encoding α1L-adrenoceptor exist. Also in human, α1-adrenoceptor of iris dilator is characterized as α1L-adrenoceptor (pA2: prazosin 7.3) (Ishikawa et al., 1996). Therefore, rabbit may be the good strain for the drug research which is relevant to human ocular therapeutics. For example, α1-adrenoceptor antagonists which have high affinity to the iris dilator may be available to reverse the effect of mydriasis rapidly and safely after diagnostic mydriasis (Mapstone, 1977; Relf et al., 1988; Allinson et al., 1990). Further study may reveal subtypes of α1-adrenoceptor in ocular tissues with different locations and profiles for various agonists and antagonists, leading to development of a subtype selective drugs with therapeutic advantages.
Acknowledgments
The authors would like to thank Ms N. Aoki for secretarial assistance and Ms N. Satio for technical assistance. This work is supported in part by grant from the Smoking Research Foundation of Japan and by Grant-in-Aid for Scientific Research (09273105, 09470023) from the Ministry of Education, Science, Sports and Culture of Japan.
Abbreviations
- CHO
Chinese hamster ovary
- RT–PCR
reverse transcription-polymerase chain reaction
- TES
transmural electrical stimulation
References
- ALLINSON R.W., GERBER D.S., BIEBER S., HODES B.L. Reversal of mydriasis by dapiprazole. Ann. Ophthalmol. 1990;22:131–138. [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. Chemother. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADFORD M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BRON A.J., TRIPATHI R.C., TRIPATHI B.J.The iris Woiff's anatomy of the eye and orbit 1997London: Chapman & Hall; 308–334.Eighth edition. ed. Bron, A.J., Tripathi, R.C. & Tripathi, B.J. pp [Google Scholar]
- BURT R.P., CHAPPLE C.R., MARSHALL I. Evidence for a functional α1A- (α1c-) adrenoceptor mediating contraction of the rat epididymal vas deferens and an α1B-adrenoceptor mediating contraction of the rat spleen. Br. J. Pharmacol. 1995;115:467–475. doi: 10.1111/j.1476-5381.1995.tb16356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BYLUND D.B., EIKENBURG D.C., HIEBLE J.P., LANGER S.Z., LEFKOWITZ R.J., MINNEMAN K.P., MOLINOFF P.B., RUFFOLO R.R.J., TRENDELENBURG U. International union of pharmacology nomenclature of adrenoceptors. Pharmacol. Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- CHANG D.J., CHANG T.K., YAMANISHI S.S., SALAZAR F.H.R., KOSAKA A.H., KHARE R., BHAKTA S., JASPER J.R., SHIEH I., LESNICK J.D., FORD A.P.D.W., DANIELS D.V., EGLEN R.M., CLARKE D.E., BACH C., CHAN H.W. Molecular cloning, genomic characterization and expression of novel human α1A-adrenoceptor isoforms. FEBS Letts. 1998;442:279–283. doi: 10.1016/s0014-5793(98)00024-6. [DOI] [PubMed] [Google Scholar]
- CHENG Y., PRUSOFF W.H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- CHIANG J., HERMODSSON G., ØIE S. The effect of α1-acid glycoprotein on the pharmacological activity of α1-adrenergic antagonists in rabbit aortic strips. J. Pharm. Pharmacol. 1991;43:540–547. doi: 10.1111/j.2042-7158.1991.tb03533.x. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- CHOPPIN A., EGLEN R.M., HEGDE S.S. Pharmacological characterization of muscarinic receptors in rabbit isolated iris sphincter muscle and urinary bladder smooth muscle. Br. J. Pharmacol. 1998;124:883–888. doi: 10.1038/sj.bjp.0701920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLAVAHAN N.A., VANHOUTTE P.M. α-Adrenoceptor classification in vascular smooth muscle. Trends Pharmacol. Sci. 1986;7:347–349. [Google Scholar]
- FORD A.P.D.W., ARREDONDO N.F., BLUE D.R., BONHAUS D.W., JASPER J., KAVA M.S., LESNICK J., PFISTER J.R., SHIEH I.A., WILLIAMS T.J., MENEAL J.E., STAMEY T.A., CLARKE D.E. RS-17053, a selective α1A-adrenoceptor antagonist, displays low affinity for functional α1-adrenoceptors in prostate of man; implications for adrenoceptor classification. Mol. Pharmacol. 1996;49:209–215. [PubMed] [Google Scholar]
- FORD A.P.D.W., BERGE N.V., CLARKE D.E.Characterization of α1-adrenoceptors in isolated anococcygeus muscle of the rat Br. J. Pharmacol. 1993109Suppl112PProc [Google Scholar]
- FORD A.P.D.W., DANIELS D.V., CHANG D.J., GEVER J.R., JASPER J.R., LESNICK J.D., CLARKE D.E. Pharmacological pleiotropism of the human recombinant α1A-adrenoceptor. Br. J. Pharmacol. 1997;121:1127–1135. doi: 10.1038/sj.bjp.0701207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORD A.P.D.W., WILLIAMS T.J., BLUE D.R., CLARKE D.E. α1-Adrenoceptor classification: sharpening Occam's razor. Trends Pharmacol. Sci. 1994;15:167–170. doi: 10.1016/0165-6147(94)90136-8. [DOI] [PubMed] [Google Scholar]
- GREEN K., HATCHETT T.L. Regional ocular blood flow after chronic topical glaucoma drug treatment. Acta. Ophthalmol. Copenh. 1987;65:503–506. doi: 10.1111/j.1755-3768.1987.tb07030.x. [DOI] [PubMed] [Google Scholar]
- HIEBLE J.P., BYLUND D.B., CLARKE D.E., EIKENBURG D.C., LANGER S.Z., LEFKOWITZ R.J., MINNEMAN K.P., RUFFOLO R.R.J. Recommendation for nomenclature of α1-adrenoceptors; Consensus update. Pharmacol. Rev. 1995;47:267–270. [PubMed] [Google Scholar]
- HIRASAWA A., SHIBATA K., HORIE K., TAKEI Y., OBIKA K., TANAKA T., MURAMOTO N., TAKAGAKI K., YANO J., TSUJIMOTO G. Cloning, functional expression and tissue distribution of human α1C-adrenoceptor splice variants. FEBS Letts. 1995;363:256–260. doi: 10.1016/0014-5793(95)00330-c. [DOI] [PubMed] [Google Scholar]
- HOLCK C.M., JONES C.H.M., HAEUSLER G. Differential interactions of clonidine and methoxamine with postsynaptic α-adrenoceptors of rabbit main pulmonary artery. J. Cardiovasc. Pharmacol. 1983;5:240–248. doi: 10.1097/00005344-198303000-00013. [DOI] [PubMed] [Google Scholar]
- HONDA K., MIYATA-OSAWA A., TAKENAKA T. α1-Adrenoceptor subtype mediating contraction of the smooth muscle in the lower urinary tract and prostate of rabbits. Naunyn-Schmiedeberg's Arch. Pharmacol. 1985;330:16–21. doi: 10.1007/BF00586704. [DOI] [PubMed] [Google Scholar]
- HONG S.J., WU K.Y., CHEN I.J. Ocular hypotensive and vasodilative effects of two β-adrenergic blockers with intrinsic sympathomimetic activity. Curr. Eye Res. 1998;17:700–707. [PubMed] [Google Scholar]
- ISHIKAWA H., MILLER D.D., PATIL P.N. Comparison of post-junctional α-adrenoceptors in iris dilator muscle of humans, and albino and pigmented rabbits. Naunyn-Schmiedeberg's Arch. Pharmacol. 1996;354:765–772. doi: 10.1007/BF00166903. [DOI] [PubMed] [Google Scholar]
- KENNY B.A., CHALMERS D.H., PHILPOTT P.C., NAYLOR A.M. Characterization of an α1D-adrenoceptor mediating the contractile response of rat aorta to noradrenaline. Br. J. Pharmacol. 1995;115:981–986. doi: 10.1111/j.1476-5381.1995.tb15907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONNO F., TAKAYANAGI I. Characterization of postsynaptic alpha1-adrenoceptors in the rabbit iris dilator smooth muscle. Naunyn-Schmiedeberg's Arch. Pharmacol. 1986;333:271–276. doi: 10.1007/BF00512940. [DOI] [PubMed] [Google Scholar]
- KRUPIN T., FEITL M., BECKER B. Effect of prazosin on aqueous humor dynamics in rabbits. Arch. Ophthalmol. 1980;98:1639–1642. doi: 10.1001/archopht.1980.01020040491021. [DOI] [PubMed] [Google Scholar]
- LEFKOWITZ R.J., CARON M.G. Adrenergic receptors. Models for the study of receptors coupled to guanine nucleotide regulatory proteins. J. Biol. Chem. 1988;263:4993–4996. [PubMed] [Google Scholar]
- LEONARDI A., HIEBLE J.P., GUARNERI L., NASELSKY D.P., POGGESI E., SIRONI G., SULPIZIO A.C., TESTA R. Pharmacological characterization of the uroselective Alpha-1 antagonist Rec 15/2739 (SB 216469) J. Pharmacol. Exp. Ther. 1997;281:1272–1283. [PubMed] [Google Scholar]
- MALMFORS T. The adrenergic innervation of the eye as demonstrated by fluorescence microscopy. Acta. Physiol. Scand. 1965;65:259–267. doi: 10.1111/j.1748-1716.1965.tb04269.x. [DOI] [PubMed] [Google Scholar]
- MAPSTONE R. Dilating dangerous pupils. Br. J. Ophthalmol. 1977;61:517–524. doi: 10.1136/bjo.61.8.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSHALL I., BURT R.P., GREEN G.M., HUSSAIN M.B., CHAPPLE C.R. Different subtypes of α1A-adrenoceptor mediating contraction of rat epididimal vas deferens, rat hepatic portal vein and human prostate distinguished by the antagonist RS 17053. Br. J. Pharmacol. 1996;119:407–415. doi: 10.1111/j.1476-5381.1996.tb16001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGREGOR M.K.L.Autonomic drugs Havener's ocular pharmacology 1994St. Louis: The C.V. Mosby Company; 53–84.ed. Mauger, T.F. & Craig, E.L. pp [Google Scholar]
- MINNEMAN K.P. α1 Adrenergic receptor subtypes, inositolphosphates and sources of cell calcium. Pharmacol. Rev. 1988;40:87–119. [PubMed] [Google Scholar]
- MITTAG T.W., TORMAY A. Adrenergic receptor subtypes in rabbit iris-ciliary body membranes: Classification by radioligand studies. Exp. Eye. Res. 1985;40:239–249. doi: 10.1016/0014-4835(85)90009-0. [DOI] [PubMed] [Google Scholar]
- MIYAMOTO S., TANIGUCHI T., SUZUKI F., TAKITA M., KOSAKA N., NEGORO E., OKUDA T., KOSAKA H., MURATA S., NAKAMURA S., AKAGI Y., OSHITA M., WATANABE Y., MURAMATSU I. Cloning, functional expression and tissue distribution of rabbit α1a-adrenoceptor. Life Sci. 1997;60:2069–2074. doi: 10.1016/s0024-3205(97)00194-x. [DOI] [PubMed] [Google Scholar]
- MURAMATSU I.A pharmacological perspective of α1-adrenoceptors: subclassification and functional aspects α-Adrenoceptors: Signal Transduction, Ionic Channels, and Effector Organs 1992Tokyo: Excerpta Medica, Ltd; 193–202.ed. Fujiware, M., Sugimoto, T. & Kogure, K. pp [Google Scholar]
- MURAMATSU I., KIGOSHI S., ODA Y. Evidence for sympathetic, purinergic transmission in the iris dilator muscle of the rabbit. Jpn. J. Pharmacol. 1994;66:191–193. doi: 10.1254/jjp.66.191. [DOI] [PubMed] [Google Scholar]
- MURAMATSU I., MURATA S., ISAKA M., PIAO H., ZHU J., SUZUKI F., MIYAMOTO S., OSHITA M., WATANABE Y., TANIGUCHI T. α1-Adrenoceptor subtypes and two receptor systems in vascular tissues. Life Sci. 1998;62:1461–1465. doi: 10.1016/s0024-3205(98)00090-3. [DOI] [PubMed] [Google Scholar]
- MURAMATSU I., OHMURA T., KIGOSHI S., HASHIMOTO S., OSHITA M. Pharmacological subclassification of α1-adrenoceptors in vascular smooth muscle. Br. J. Pharmacol. 1990;99:197–201. doi: 10.1111/j.1476-5381.1990.tb14678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURAMATSU I., OSHITA M., YAMANAKA K. Selective alpha-2 blocking action of DG-5128 in the dog mesenteric artery and rat vas deferens. J. Pharmacol. Exp. Ther. 1983;227:194–198. [PubMed] [Google Scholar]
- MURATA S., TANIGUCHI T., MURAMATSU I. Pharmacological analysis of the novel, selective α1-adrenoceptor antagonist, KMD-3212 and its suitability as a tritiated radioligand. Br. J. Pharmacol. 1999;127:19–26. doi: 10.1038/sj.bjp.0702489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARITA S., WATANABE M. Responses of isolated rat iris dilator to adrenergic and cholinergic agents and electrical stimulation. Life Sci. 1982;30:1211–1218. doi: 10.1016/0024-3205(82)90665-8. [DOI] [PubMed] [Google Scholar]
- NISHIMURA K., KUWAYAMA Y., MATSUGI T., SUN N., SHIRASAWA E. Selective suppression by bunazosin of alpha-adrenergic agonist evoked elevation of intraocular pressure in sympathectomized rabbit eyes. Invest. Ophthalmol. Vis. Sci. 1993;34:1761–1766. [PubMed] [Google Scholar]
- QIN M., ØIE S. Dose α1-acid glycoprotein act as a non-functional receptor for α1-adrenergic antagonists. J. Pharm. Pharmacol. 1994;46:896–901. doi: 10.1111/j.2042-7158.1994.tb05710.x. [DOI] [PubMed] [Google Scholar]
- RELF S.J., GHARAGOZLOO N.Z., SKUTA G.L., ALWARD W.L., ANDERSON D.R., BRUBAKER R.F. Thymoxamine reverses phenylephrine-induced mydriasis. Am. J. Ophthalmol. 1988;106:251–255. doi: 10.1016/0002-9394(88)90356-x. [DOI] [PubMed] [Google Scholar]
- SCHWIETERT H.R., GOUW M.A., WILHELM D., WILFFERT B., VAN ZWIETEN P.A. The role of α1-adrenoceptor subtypes in the phasic and tonic responses to phenylephrine in the longitudinal smooth muscle of the rat portal vein. Naunyn-Schmiedeberg's Arch. Pharmacol. 1991;343:463–471. doi: 10.1007/BF00169547. [DOI] [PubMed] [Google Scholar]
- SHIBATA K., FOGLAR R., HORIE K., SAKAMOTO A., OGAWA S., TSUJIMOTO G. KMD-3213, a novel, potent, α1a-adrenoceptor-selective antagonist: Characterization using recombinant human α1-adrenoceptors and native tissues. Mol. Pharmacol. 1995;48:250–258. [PubMed] [Google Scholar]
- SUZUKI F., MIYAMOTO S., TAKITA M., OSHITA M., WATANABE Y., KAKIZUKA A., NARUMIYA S., TANIGUCHI T., MURAMATSU I. Cloning, functional expression and tissue distribution of rabbit α1d-adrenoceptor. Biochim. Biophys. Acta. 1997;1323:6–11. doi: 10.1016/s0005-2736(96)00229-5. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI M., TANIGUCHI T., MURATA S., OKADA K., MORIYAMA N., YAMAZAKI S., MURAMATSU I.New α1-adrenoceptor antagonist, JTH-601, shows more than 10 times higher affinity for the human prostates than arteries J. Urol. 1998161in press [PubMed] [Google Scholar]
- VAN ALPHEN G.W.H., KERN R., ROBINETTE S.L. Adrenergic receptors of the intraocular muscles. Arch. Ophthalmol. 1965;74:253–259. doi: 10.1001/archopht.1965.00970040255025. [DOI] [PubMed] [Google Scholar]
- WILLIAMS T.J., CLARKE D.E.α1-Adrenoceptors mediating norepinephrine contraction of the rabbit prostate in vitro Br. J. Pharmacol. 1993108161PProc. Suppl [Google Scholar]
- WILLIAMS T.J., CLARKE D.E., FORD A.P.D.W.Whole-cell radioligand binding assay reveals α1L-adrenoceptor antagonist profile for the human cloned α1A-adrenoceptor in CHO-K1 cells Br. J. Pharmacol. 1996119359PProc. Suppl [Google Scholar]
- YAMAGISHI R., AKIYAMA K., NAKAMURA S., HORA M., MASUDA N., MATSUZAWA A., MURATA S., UJIIE A., KURASHINA Y., IIZUKA K., KITAZAWA M. Effect of KMD-3213, an α1a-adrenoceptor-selective antagonist, on the contractions of rabbit prostate and rabbit and rat aorta. Eur. J. Pharmacol. 1996;315:73–79. doi: 10.1016/s0014-2999(96)00589-4. [DOI] [PubMed] [Google Scholar]
- YAMANAKA K., KIGOSHI S., MURAMATSU I. The selectivity of DG-5128 as an alpha 2-adrenoceptor antagonist. Eur. J. Pharmacol. 1984;106:625–628. doi: 10.1016/0014-2999(84)90068-2. [DOI] [PubMed] [Google Scholar]


