Abstract
Experiments were carried out to explore the possible role played by the nitric oxide (NO) system in the organum vasculosum laminae terminalis (OVLT) of rat brain in arterial pressure regulation.
Intracerebroventricular (ICV) or intra-OVLT administration of NO donors such as hydroxylamine, sodium nitro-prusside or s-nitro-acetylpenicillamine caused an up to 55 mmHg decrease in blood pressure (BP) but an increase in NO release (measured by porphyrin/nafion coated carbon fibre electrodes in combination with voltammetry) in the OVLT. In contrast, ICV or intra-OVLT administration of NG-nitro-L-arginine methyl ester (L-NAME; a constitutive NO synthase inhibitor) caused an up to 45 mmHg increase in BP but a fall in NO release in the OVLT.
Compared with the BP responses induced by ICV injection of NO donors or NO synthase inhibitors, the OVLT route of injection required a much lower dose of NO donors or NO synthase inhibitors to produce a similar BP effect.
The depressor effects induced by ICV or intra-OVLT administration of NO donors were attenuated by pretreatment with intra-OVLT injection of methylene blue (an inhibitor of guanylate cyclase), haemoglobin (a NO scavenger), L-NAME or spinal transection. On the other hand, the L-NAME-induced pressor effects were attenuated by pretreatment with intra-OVLT injection of L-arginine or spinal transection.
The data suggest that activation of cyclic GMP-dependent NO synthase in the OVLT of rat brain causes cyclic GMP-dependent decreases in arterial pressure via inhibiting the sympathetic efferent activity.
Keywords: Organum vasculosum laminae terminalis, voltammetry, sympathetic tone, blood pressure
Introduction
Increasing evidence indicates that nitric oxide (NO) plays an important role in central regulation of blood pressure (BP) and sympathetic tone (Dinerman et al., 1993; Horn et al., 1994; Sakuma et al., 1992; Shapoval et al., 1991; Togashi et al., 1992; Tramu et al., 1983; Zangiger et al.,1995). Relatively large numbers of NO-producing neurons are found in the paraventricular nuclei (PVN) and supraoptic nuclei of the hypothalamus (Bredt et al., 1990; Horn et al., 1994; Miyagawa et al., 1994; Sanchez et al., 1994; Vincent & Kimura, 1992), the nucleus of the tractus solitarius (NTS) and the ventrolateral medulla (Dun et al., 1994; 1995; Sanchez et al., 1994), and the organum vasculosum laminae terminalis (OVLT) (Jurzak et al., 1994). Microinjection of NO donors into the NTS (Lewis et al., 1991) or the PVN (Horn et al., 1994) has been reported to elicit significant decreases in BP of rats. On the other hand, administration of NO synthase (NOS) inhibitors into the rostral ventrolateral medulla (Shapoval et al., 1991) or NTS (Harada et al., 1993) increases BP of rats. These observations emphasize the potential of NO to act within NTS, PVN or rostral medulla to influence central control of the cardiovascular system.
Many studies have also indicated that the OVLT is a major forebrain area for maintaining the homeostasis of blood pressure (Johnson & Gross, 1993). For example, electrical or chemical stimulation of this area increases arterial blood pressure while chemical lesions of this area attenuates the angiotensin II-induced pressor effects (Bellin et al., 1987; 1988; Mangiapane & Brody, 1997). However, despite the anatomical demonstration of NOS within OVLT, there is to date no direct evidence addressing the potential cardiovascular roles of NO within this nucleus.
We have therefore undertaken to assess the effects of central administration of several NO donors or NOS inhibitors on both the cardiovascular responses and the NO formation in the OVLT of rat brain. Prophyrin/nafion coated carbon fibre micro-electrodes in combination with in vivo voltammetry were used to determine the brain NO formation.
Methods
Experimental animals
Two-hundred-and-forty-two male Sprague-Dawley rats (250–350 g) were used in the entire series of experiments. Upon receipt from the supplier (Animal Resource Center, National Cheng Kung University Medical College, Tainan City, Taiwan, Republic of China), the animals were housed in a temperature-regulated (22±1°C) room on 12/12 h light/dark cycle with food and water ad libitum for at least 2 weeks before experiments. The light was turned on at 0600 h and turned off at 1800 h.
Surgical preparation
The animals were anaesthetized with urethane (1.4 g kg−1, i.p.) and placed in a Kopf stereotaxic apparatus. For direct injection of drugs into the lateral cerebral ventricle or the OVLT, a stainless-steel cannula which consisted of a guide tube (0.81 mm outer diameter) with a snugly fitting trocar was implanted into the lateral cerebral ventricle (AP, −0.8 mm; LAT, −1.5 mm and DV, −3.5 mm) or the OVLT (AP, −0.5 mm; LAT, −0.1 mm and DV, −8.5 mm) according to the atlas and the coordinates of Paxinos & Watson (1982). Microinjection was made into the OVLT through a 26 gauge cannula connecting to a 10-μ1 Hamilton microsyringe. The volume of fluid injected over 5 s was 5.0 or 0.5 μl for intracerebroventricular (ICV) or intra-OVLT injection, respectively. For measurement of NO release, a porphyrin/nafion-coated carbon fibre electrode was implanted stereotaxically into the OVLT. Auxiliary (silver wire) and reference (Ag/AgCl) electrodes were placed on the dura surface of the parietal skull. Differential pulse voltammograms were then recorded automatically every 0.5 s. For assessment of cardiovascular functions, a fine catheter was inserted into the femoral artery and was connected via a Statham blood pressure transducer to a Gould 4-channel polygraph for recording mean and pulsatile arterial blood pressure. Both the heart rate and blood pressure were measured. In preparing spinal transection, the cervical vertebrae were exposed and complete transection was made with a spatula at the seventh cervical segment of the spinal cord.
Drugs
Drugs, administered into the OVLT included hydroxylamine (Sigma; 0.01–0.1 mg), sodium nitroprusside (SNP; sigma; 0.01–0.1 mg), S-nitroso-acetylpenicillamine (SNAP; RBI; 0.25 mg), sodium azide (NaN3; Sigma; 0.1 mg), NG-nitro-L-arginine methyl ester (L-NAME; RBI; 0.05–0.2 mg), methylene blue (Sigma; 0.05 mg), 8-bromo-cyclic GMP (Sigma; 0.05 mg), hemoglobin (Sigma; 100 μg) and aminoguanidine (1 mg). SNAP was dissolved in DMSO solution and diluted to 5% with saline. All other compounds were dissolved in saline. The drugs administered intracerebroventricularly included hydroxylamine (0.125–0.5 mg) or L-NAME (0.1–1.0 mg). Different dose experiments were repeated in same animals at an interval of 60–90 min between injection.
NO monitoring
A multiple carbon fibre (28 μm in diameter, AVCO, Lowell, MA, U.S.A.) was inserted into the pulled glass micropipette (20–25 mm in length). The tip was cut, then carbon fibre was pushed out of the pipette tip. Electrical contact with the fibre was made using silver paste. The tip and blunt end of the pipet were sealed with cyanoacrylate adhesive (super glue). The entire surface of a pyrolytic carbon fibre was 12 μm thick and 100±25 μm long. To improve the sensitivity and selectivity of carbon fibre for NO, the electrode was electrically pretreated as described previously (Lin et al., 1995; 1997). This treatment consisted of a DC current applied in two stages, 2.2 V for 30 s in 0.1 M H2SO4, and 2.2 V for 30 s in 0.1 M HCl. The carbon fibre electrode was washed with distilled water. The tip of the carbon fibre electrode was coated with nickel(II) tetrakis (3-methoxy-4-hydroxyphenyl)-porphyrin by use of cyclic scanning at a potential between −0.2 and 1.0 V. The polymeric porphyrin was subsequently coated by dipping for 10 s in 1% nafion solution (Aldrich Chemical Company, Inc, Milwaukee, WI., U.S.A.). The porphyrin/nafion-coated electrode was then dried at 60°C for 20 s and used immediately for in vitro followed by in vivo measurements. Differential pulse amperometry was performed in vitro and in vivo with a Biopulse (Solea Tacussel Co., France) using the following scan parameters: imposed initial potential=−220 mV; imposed final potential=+690 mV; prepulse=100 ms; measuring pulse=60 ms; measuring potential=50 mV; and pulse cycle=0.5 s. The sensitivity of the porphyrin/nafion-coated carbon fibre electrode to several NO donors including hydroxylamine (1–1000 μM), SNP (100 μM), NaNO2 (100 μM), HNO3 (100,1000 μM), or NaN3 (100 μM) was determined using differential pulse amperometry in a temperature-controlled (37°C) water bath. Phosphate buffered saline (0.1 M, pH 7.4) was used as blank and solvent for the test solution. To determine the sensitivity of the porphyrin/nafion-coated electrodes (resistance=200 Ω; capacitance=0.0056 μF) for NO over NO2, NO3, ascorbic acid, dopamine, or serotonin, a ratio of the sensitivity of hydroxylamine/NaNO2, hydroxylamine/HNO3, hydroxylamine/dopamine, or hydroxylamine/serotonin was calculated. Our electrodes were three times or >1000 times more sensitive to hydroxylamine than to NaNO2 or to HNO3, ascorbic acid. Our electrodes are insensitive to dopamine, or serotonin.
Histology
After the completion of the experiments, an aliquot of 0.5 μl of methylene blue was injected down the cannula to measure the spread of the injected solution. The head of each animal was perfused with PBS solution, followed by 4% paraformaldehyde (PFA) fixative solution. After perfusion, the brain was removed and placed in a well labelled glass vial filled with 4% PFA fixative, and fixed at 4°C for 0.5–1 h after which the solution was changed to 15% sucrose in PBS until the brain sank in the vial. Later, the fixed brains were cut in 50 μm sections so that stereotaxic coordinates of injection site in each animal were verified. It was found that the stained cross-sectional area in the OVLT was approximately 0.5 mm in diameter.
Statistics
Data obtained from 238 animals were included after successful experiments including histological verification of the stereotaxic target. Blood pressure and NO release responses were assessed as changes from pre-injection values (mmHg or nM). Results are expressed as the mean±s.e.mean for n experiments. A two way analysis of variance (ANOVA) with repeated measures was used to evaluate the difference between groups. Differences between individual means were assessed by post hoc Scheff's multiple range tests. P<0.05 was taken to indicate statistical significance.
Results
Studies with NO donors
Intracerebroventricular administration of hydroxylamine caused a dose-dependent decrease in the mean arterial pressure (MAP) but an increase in NO release from the OVLT of rat brain. The data are summarized in Figure 1. Figure 2 depicts tracings from a representative experiment showing the effect of ICV injection of hydroxylamine on pulsatile BP, MAP and NO release of the OVLT. Direct administration of hydroxylamine, SNP, NaN3 or L-arginine into the OVLT of rat brain (Table 1) or ICV injection of L-arginine (Table 2) also caused a decrease in MAP. Compared with the MAP responses induced by ICV injection of NO donors, the OVLT route of injection required a much lower dose of NO donors to produce a similar depressor effect. As shown in Figure 3, compared to those caused by intra-OVLT injection of hydroxylamine, administration of same amount of hydroxylamine into the neighbouring areas of the OVLT caused decreased MAP changes.
Figure 1.

Effects of intracerebroventricular administration of hydroxylamine on NO release in the OVLT and MAP in rats. Data represent mean±s.e.mean of eight rats. *P<0.05, significantly different from the control values (saline group), ANOVA.
Figure 2.

Representative tracings showing the effects of administration of hydroxylamine into the lateral ventricle (ICV) on NO release recorded in the OVLT area, blood pressure (BP) and mean arterial pressure (MAP) in a rat. The basal level of NO in the OVLT is 325 nM.
Table 1.
Maximal changes in mean arterial pressure (MAP) produced by intra-OVLT administration of NO donors, NOs inhibitors or NO scavenger rats

Table 2.
Maximal changes in mean arterial pressure (MAP) produced by intracerebroventricular (ICV) administration of NO donors and intra-OVLT administration of a NO scavenger in rats

Figure 3.
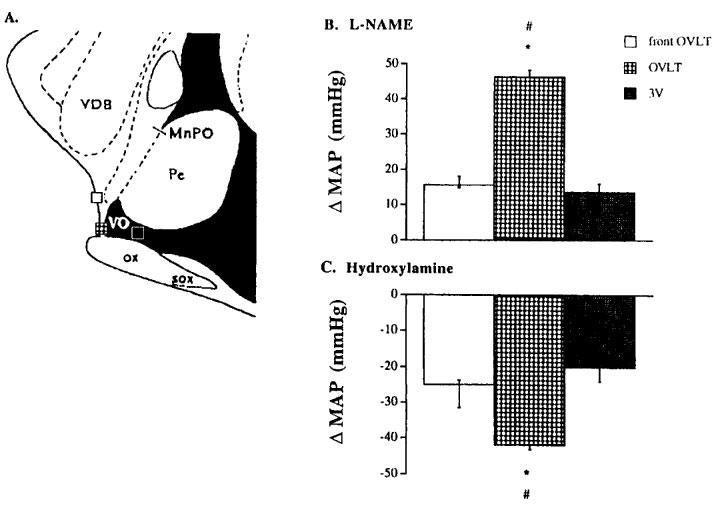
(A) Mid-sagittal section of the rat upper brain stem showing the sites at which L-NAME and hydroxylamine were administered into the OVLT, the third ventricle (3V) and the front OVLT region taken from Paxinos & Watson (1982). (B) MAP changes caused by microinjection of L-NAME (0.5 mg) into the three different brain regions; and (C) MAP changes caused by microinjection of hydroxylamine (0.1 mg) into the three different brain regions. In both (B) and (C), data represent mean±s.e.mean of eight rats. #, *P<0.05, significantly different from the control values (either front OVLT or 3V group respectively), ANOVA. VDB, nucleus ventrical limb digonal band; ac, anterior commissure; MnPO, median preoptic nucleus; PE, periventricular hypothalamic nucleus; OX, optic chiasma; VO, vascular organ; SOX, supraoptic decussation.
Studies with NOS inhibitors
In contrast, ICV administration of L-NAME caused a dose-dependent increase in MAP but a decrease in NO release in the OVLT of rat brain. The data are summarized in Figure 4. Figure 5 depicts tracings from a typical experiment showing the effect of ICV injection of L-NAME on the pulsatile BP, MAP and NO release of the OVLT. Direct administration of L-NAME, but not another NOS inhibitor aminoguanidine, into the OVLT also caused an increase in MAP (Table 1). Compared to those produced by ICV injection of L-NAME, the OVLT route of injection required a much lower dose of L-NAME. In addition, compared to those produced by intra-OVLT dose of L-NAME, administration of the same dose of L-NAME into any one of neighbouring areas of the OVLT caused decreased MAP responses (Figure 3). Pretreatment of rats with intra-OVLT injection of L-NAME significantly attenuated the MAP responses induced by ICV (Table 2) or intra-OVLT (Table 3) injection of either hydroxylamine or L-arginine.
Figure 4.

Effects of intracerebroventricular administration of L-NAME on NO release in the OVLT and MAP in rats. Data represent mean±s.e.mean of eight rats. *P<0.05, significantly different from the control values (saline group), ANOVA.
Figure 5.

Representative tracings showing the effects of administration of L-NAME into the lateral ventricle (lv) on NO release recorded in the OVLT area, blood pressure (BP) and mean arterial pressure (MAP) in a rat. The basal level of NO in the OVLT is 338 nM.
Table 3.
Maximal changes in mean arterial pressure (MAP) produced by intra-OVLT administration of NO donors or NOS inhibitors in rats

Studies with a cyclic GMP analogue
Intra-OVLT administration of the membrane permeable analogue of cyclic GMP, 8-Bromo-cyclic GMP (0.05 mg), produced a decrease in MAP (Table 1).
Studies with a cyclic GMP inhibitor or a NO scavenger
Pretreatment of rats with intra-OVLT injection of methylene blue (an inhibitor of soluble guanylate cyclase, 0.05 μg, n=6) or hemoglobin (a NO scavenger; 100 μg, n=6), although showing no effect on the basal levels of MAP, significantly attenuated the depressor effects induced by intra-OVLT (Table 3) or ICV (Table 2) injection of hydroxylamine.
Studies with spinal transection
When hydroxylamine or L-NAME was administered into the OVLT of the rats decentralized by spinal cord transection at the C7 level, changes in MAP were decreased as compared to those of the sham-operated controls (Table 4).
Table 4.
Maximal changes in MAP produced by intra-OVLT administration of hydroxlamine or L-NAME in sham-operated and spinal transected rats

Discussion
In the present study, in vivo voltammetry was used in combination with electrochemically treated prophyrin/nafion coated carbon fibre electrodes for measuring the NO release in the OVLT of rat brain. Direct administration of hydroxylamine (a NO donor) into the cerebroventricular fluid system elicits a decrease in MAP but an increase in NO release in the OVLT of rat brain. In contrast, ICV administration of L-NAME (a NOS inhibitor) elicits an increase in MAP but a decrease in OVLT NO release. The BP responses caused by ICV injection of either hydroxylamine or L-NAME could be mimicked by direct administration of hydroxylamine or L-NAME into the OVLT area of rat brain, respectively. Compared to the BP responses induced by ICV injection of NO donors or NOS inhibitors, the OVLT route of injection required a much lower dose of NO donors or NOS inhibitors to produce a similar BP response. In addition, intra-OVLT administration of other NO donors (including SNP, SNAP, NaN3 or L-arginine) also caused a decrease in BP. Furthermore, microinjection of specific antagonists of NOS lead to an inhibition of the pressure effects of intraventricular injection of NO donors. These results suggest that the rise and the fall in BP relate to the decrease and the increase in NO release in the OVLT, respectively. The contention is supported by several previous results. For example, the positive identification of NOS in the OVLT area was demonstrated by Jurzak and his colleagues (1994). Electrical or chemical stimulation of the OVLT also elicited an increase in BP (Mangiapane & Brody, 1997).
In fact, three different isoforms of NOS have been described. Two constitutive forms producing NO have been found in endothelial (eNOS) and neuronal (nNOS) cells (Misko et al., 1993). A cytokine inducible form of NOS (iNOS) can be expressed in vascular smooth muscle cells, endothelium cells, muscle cells, macrophages, hypothalamus, and pituitary (Brann et al., 1997; Satta et al., 1998). L-NAME and L-NMMA are equally potent inhibitors of eNOS from porcine endothelial cells (McCall et al., 1991). Aminoguanidine is more than 20 fold less potent than L-NMMA on the activity of nNOS purified from rat cerebellum (McCall et al., 1991) and 40 fold less potent than L-NMMA in blocking eNOS mediated vasodilation in rats (Corbett et al., 1992). In contrast, aminoguanidine is a 2 fold more potent inhibitor of iNOS from endotoxin-induced RAW 264.7 macrophages than L-NMMA (Misko et al., 1993), and L-NAME is a 3–5 fold less potent inhibitor of iNOS in the murine macrophage cell-line J774 compared to L-NMMA (Mayer et al., 1993). In this study, intra-OVLT administration of L-NAME, but not aminoguanidine, elicits an increase in MAP in rats. Given the high degree of vascularization of the OVLT, BP effects could be due to NO of endothelial origin (Jurzak et al., 1994). These observations suggest that NO produced by constitutive, rather than inducible NOS, in the OVLT of rat brain mediates arterial pressure regulation.
In the present results, the hydroxylamine-induced depressor response or the L-NAME-induced pressor response was, respectively, attenuated by pretreatment with intra-OVLT injection of haemoglobin (a NO scavenger) (Kanner et al., 1992) or L-arginine. In addition, the present results show that pretreatment with intra-OVLT injection of methylene blue, an inhibitor of NOS and soluble guanylate cyclase (Ignarro et al., 1984; McCall et al., 1991), attenuates the depressor effects induced by intra-OVLT or ICV administration of hydroxylamine. This suggests that the NOS-dependent production of cyclic GMP in the OVLT is involved in depressor responses. Indeed, the present results show that intra-OVLT injection of 8-bromo-cyclic GMP, the membrane permeable analogue of cyclic GMP, also causes a depressor response.
The present results further demonstrate that the depressor effects caused by intra-OVLT administration of hydroxylamine as well as the pressor effects caused by intra-OVLT administration of L-NAME was attenuated by pretreatment with spinal transection. These results imply that the decrease of arterial pressure after intra-OVLT administration of hydroxylamine or the increase of arterial pressure after intra-OVLT administration of L-NAME in sham-operated rats are attributable to inhibition or activation of the sympathetic efferent pathway, respectively. In fact, previous results have also shown that administration of NO donors into any one of the autonomic sites (e.g. PVN or NTS) in brain elicits significant changes in BP of rats (Harada et al., 1993; Horn et al., 1994). It would appear most likely that NO action affects the normal intrinsic tonic activity of any one of these groups (e.g. OVLT, PVN or NTS) of neurons (that exert direct control over sympathetic preganglionic neurons in the intermediolateral cell column) and could explain the observed responses in BP of rats.
The inference from the present results is that many of the effects of ICV application of NO donors or NOS inhibitors are due to the release or inhibition of release of NO within the OVLT, respectively. To properly address this question, NO donors should be given ICV and a NO scavenger should be given directly into the OVLT. Indeed, in the present results, we have demonstrated that the depressor effects produced by ICV injection of NO donors can be attenuated by intra-OVLT injection of a NO scavenger in rats.
In summary, elevating NO release in the OVLT of rat brain by NO donors decreases BP, while lowering NO release in the OVLT by NOS inhibitors increases BP in rats. The depressor effects induced by ICV injection of NO donors are attenuated by intra-OVLT administration of a NO scavenger. Compared with the BP responses induced by ICV injection of NO donors or NOS inhibitors, the OVLT route of injection required a much lower dose of NO donors or NOS inhibitors to produce a similar BP effect. The depressor responses induced by NO donors can be attenuated by intra-OVLT injection of methylene blue (an inhibitor of guanylate cyclase), haemoglobin (a NO scavenger), L-NAME (a constitutive NOS inhibitor) or spinal transection. On the other hand, the pressor effects of a NOS inhibitor can be attenuated by intra-OVLT injection of L-arginine or spinal transection. The data suggest that activation of NOS in the OVLT of rat brain causes cyclic GMP-dependent decreases in BP via inhibiting the sympathetic tone in rats.
Recently, Horn and his associates (1994) have collected PVN microdialysis perfusates and measured the concentrations of specific amino acids in these samples to determine the effects of NO on their release within PVN. They provide the first evidence that NO may play significant roles in regulating central nervous system control over the cardiovascular system actions within the PVN and NO may exert significant control over endogenous release of amino acid neurotransmitters within this region of the brain. Immunocytochemical studies have reported high concentrations of NO (Jurzak et al., 1994), dopamine and serotonin (Jennes et al., 1982; Tramu et al., 1983) within OVLT. In addition, the pressor effects induced by intracerebral injection of angiotensin were attenuated by intra-OVLT administration of 6-hydroxydopamine (a neurotoxin which is able to deplete central dopamine) (Bellin et al., 1987; 1988). These observations have led to the suggestion that the NO within OVLT acts through the endogenous release of these amino acids to induce BP responses. Therefore, future studies should be undertaken to determine the effects of local administration of NO donors, NOS inhibitors, or dopamine agonists or antagonists into the OVLT on cardiovascular variables. In addition, experiments should be carried out to collect OVLT microdialysis perfusates and to measure the concentrations of dopamine in these samples to determine the effects of NO on their release within OVLT.
Acknowledgments
Financial support was provided by grants from National Science Council of Republic of China (NSC 86-2745-B010-005) and VGH-NYMU joint research program (VGHYM-86-S4-18 and 87-S4-22), Tsou's Foundation, Republic of China.
Abbreviations
- BP
blood pressure
- ICV
intracerebraoventricular
- NO
nitric oxide
- NOS
nitric oxide synthase
- NTS
the nucleus of tractus solitarius
- OVLT
organum vasculosum laminae terminalis
- PVN
paraventricular nuclei
- SNP
sodium nitro-presside
- SNAP
s-nitro-acetylpenicillamine
- L-NAME
NG-nitro-L-arginine methyl ester
- MAP
mean arterial pressure
References
- BELLIN S.I., LANDAS S.K., JOHNSON A.K. Localized injection of 6-hydroxy-dopamine into laminae terminalis-associated structures: effects on experimentally induced drinking and pressor responses. Brain Res. 1987;416:75–83. doi: 10.1016/0006-8993(87)91498-3. [DOI] [PubMed] [Google Scholar]
- BELLIN S.I., LANDAS S.K., JOHNSON A.K. Selective catecholamine depletion of structures along the ventral laminae terminalis: effects on experimentally induced drinking and pressor responses. Brain Res. 1988;45:9–16. doi: 10.1016/0006-8993(88)90340-x. [DOI] [PubMed] [Google Scholar]
- BRANN D.W., BHAT G.K., LAMAR C.A., MAHESH V.B. Gaseous transmitters and neuroendocrine regulation. Neuroendocrinology. 1997;65:385–395. doi: 10.1159/000127201. [DOI] [PubMed] [Google Scholar]
- BREDT D.S., HWANG P.M., SNYDER S.H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- CORBETT J.A., TILTON R.G., CHANG K., HASAN K.S., IDO Y., WANG J.L., SWEETLAND M.A., LANCANSTER J., JR, WILLIAMSON J.R., MCDANIEL M.L. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992;41:552–556. doi: 10.2337/diab.41.4.552. [DOI] [PubMed] [Google Scholar]
- DINERMAN J.L., LOWENSTEIN C.J., SNYDER S.H. Molecular mechanisms of nitric oxide regulation. Potential relevance to cardiovascular disease. Circulation. 1993;73:217–222. doi: 10.1161/01.res.73.2.217. [DOI] [PubMed] [Google Scholar]
- DUN N.J., DUN S.L., FOSTERMANN U. Nitric oxide synthase immunoreactivity in rat potine medullary neurons. Neuroscience. 1994;59:429–445. doi: 10.1016/0306-4522(94)90607-6. [DOI] [PubMed] [Google Scholar]
- DUN N.J., DUN S.L., HWANG L.L., FOSTERMANN U. Infrequent co-existence of nitric oxide synthase and parvalbumin, calbindin and calretinin immunoreactivity in rat pontine neurons. Neurosci. Lett. 1995;191:165–168. doi: 10.1016/0304-3940(95)11582-h. [DOI] [PubMed] [Google Scholar]
- HARADA S., TOKUNAGA S., MOMOHARA M., MASAKI H., TAGAWA T., IMAIZUMI T., TAKESHITA A. Inhibition of nitric oxide formation in the nucleus tractus solitarius increases renal sympathetic nerve activity in rabbits. Circ. Res. 1993;72:511–516. doi: 10.1161/01.res.72.3.511. [DOI] [PubMed] [Google Scholar]
- HORN T., SMITH P.M., MCLAUGHLIN B.E., BAUCE L., MARKS G.S., PITTMAN Q.J., FERGUSON A.V. Nitric oxide actions in paraventricular nucleus: Cardiovascular and neurochemical implications. Am. J. Physiol. 1994;266:R306–R313. doi: 10.1152/ajpregu.1994.266.1.R306. [DOI] [PubMed] [Google Scholar]
- IGNARRO L.J., BALLOT B., WOOD K.S. Regulation of soluble guanylate cyclase activity by porphyrins and metalloporphysins. J. Biol. Chem. 1984;259:6201–6207. [PubMed] [Google Scholar]
- JENNES L., BECKMAN W.C., STUMPH W.E., GRZANNA R. Anatomical relationships of serotoninergic and noradrenergic projections within the GnRH system in the septum and hypothalamus. Exp. Brain Res. 1982;46:331–338. doi: 10.1007/BF00238628. [DOI] [PubMed] [Google Scholar]
- JOHNSON A.K., GROSS P.M. Sensory circumventricular organs and brain homeostatic pathways. Fed. Am. Soc. Exp. Biol. 1993;7:678–686. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- JURZAK M., MULLER A.R., SCHMID H.A., GERSTBERGER R. Primary culture of circumventricular organs from the rat brain laminae terminalis. Brain Res. 1994;662:198–208. doi: 10.1016/0006-8993(94)90813-3. [DOI] [PubMed] [Google Scholar]
- KANNER J., HAREL S., GRANT R. Nitric oxide, an inhibitor of lipid oxidation by lipoxygenase, cyclo-oxygenase and haemoglobin. Lipids. 1992;27:46–49. doi: 10.1007/BF02537058. [DOI] [PubMed] [Google Scholar]
- LEWIS S.J., MACHADO B.H., OHTA H., TALMAN W.T. Processing of cardiopulmonary afferent input within the nucleus tractus solitarii involves activation of soluble guanylate cyclase. Eur. J. Pharmacol. 1991;203:327–328. doi: 10.1016/0014-2999(91)90737-b. [DOI] [PubMed] [Google Scholar]
- LIN A.M., KUO J.S., CHAI C.Y. Involvement of nitric oxide in dopaminergic transmission in rat striatum: an in vivo electrochemical study. J. Neurochem. 1995;65:2043–2049. doi: 10.1046/j.1471-4159.1995.65052043.x. [DOI] [PubMed] [Google Scholar]
- LIN M.T., LIN J.H., YANG Y.L. Dexamethasone administered into organum vasculosum laminae terminalis of rabbits induced anti-pyresis via inhibiting nitric oxide pathway in situ. Neurosci. Lett. 1997;230:53–56. doi: 10.1016/s0304-3940(97)00463-1. [DOI] [PubMed] [Google Scholar]
- MANGIAPANE M.I., BRODY M.L. Vasoconstrictor and vasodilator sites within anteroventral third ventricle region. Am. J. Physiol. 1997;253:R827–R831. doi: 10.1152/ajpregu.1987.253.6.R827. [DOI] [PubMed] [Google Scholar]
- MAYER B., BRUNNER F., SCHMIDT K. Inhibition of nitric oxide synthesis by methylene blue. Biochem. Pharmacol. 1993;45:367–374. doi: 10.1016/0006-2952(93)90072-5. [DOI] [PubMed] [Google Scholar]
- MCCALL T.B., FEELISCH M., PALMER R.M.J., MONCADA S. Identification of N-iminoethyl-L-ornithine as an irreversible inhibitor of nitric oxide synthase in phagocytic cells. Br. J. Pharmacol. 1991;41:552–556. doi: 10.1111/j.1476-5381.1991.tb12159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISKO T.P., MORE W.M., KASTEN T.P., NICKOLS G.A., CORBETT J.A., TILTON R.G., MCDANIEL M.L., WILLIAMSON J.R., CURRIE M.G. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur. J. Pharmacol. 1993;233:119–125. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- MIYAGAWA A., OKAMURA H., IBATA Y. Coexistence of oxytocin and NADPH-diphorase in magnocellular neurons of the paraventricular and the supraoptic nuclei of the rat hypothalamus. Neurosci. Lett. 1994;171:13–16. doi: 10.1016/0304-3940(94)90592-4. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. Academic Press; New York; 1982. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- SAKUMA I., TOGASHI H., YOSHIDA M., SAITO H., YANAGIDA M., TAMURA M., KOBAYASHI T., YASUDA H., GROSS S.S., LEVI R. N-Methyl-L-arginine, an inhibitor of L-arginine-derived nitric oxide synthesis, stimulates renal sympathetic nerve activity in vivo: a role for nitric oxide in the central regulation of sympathetic tone . Circ. Res. 1992;70:607–611. doi: 10.1161/01.res.70.3.607. [DOI] [PubMed] [Google Scholar]
- SANCHEZ F., ALONSO J.R., AREVALO R., BLANCO E., AIJON J., VANZQUEZ R. Coexistence of NADPH-diaphorase with vasopressin and oxytocin in the hypothalamic magnocellular neurosecretory nuclei of the rat. Cell Tissue Res. 1994;276:31–34. doi: 10.1007/BF00354781. [DOI] [PubMed] [Google Scholar]
- SATTA M.A., JACOBS R.A., KALTSAS G.A., GROSSMAN A.B. Endotoxin induces interleukin-1 β and nitric oxide synthase mRNA in rat hypothalamus and pituitary. Neuroendocrinology. 1998;67:109–116. doi: 10.1159/000054305. [DOI] [PubMed] [Google Scholar]
- SHAPOVAL L.N., SAGACH V.F., POBEGAILO L.S. Nitric oxide influences ventrolateral medullary mechanisms of vasomotor control in the cat. Neurosci. Lett. 1991;132:47–50. doi: 10.1016/0304-3940(91)90430-2. [DOI] [PubMed] [Google Scholar]
- TOGASHI H., SAKUMA I., YOSHIOKA M., KOBAYASHI T., YASUDA H., KITABATAKE A., SAITO H., GROSS S.S., LEVI R. A central nervous system action of nitric oxide in blood regulation. J. Pharmacol. Exp. Ther. 1992;262:343–347. [PubMed] [Google Scholar]
- TRAMU G., PILLEZ A., LEONARDELLI J. Serotonic axons of the ependyma and circumventricular organs in the forebrain of the guinea-pig. An immunohistochemical Study. Cell Tissue Res. 1983;228:297–311. doi: 10.1007/BF00204880. [DOI] [PubMed] [Google Scholar]
- VINCENT S.R., KIMURA H. Histochemical mapping of nitric oxide synthase in the rat brain. Neurosci. 1992;46:755–784. doi: 10.1016/0306-4522(92)90184-4. [DOI] [PubMed] [Google Scholar]
- ZANGIGER J., CZACHURSKI J., SELLER H. Inhibition of basal and reflex-mediated sympathetic activity in the RVLM by nitric oxide. Am. J. Physiol. 1995;268:R958–R962. doi: 10.1152/ajpregu.1995.268.4.R958. [DOI] [PubMed] [Google Scholar]


