Abstract
A novel tricyclic dinitrile, KN244, blocked the wild-type (dieldrin-sensitive) homo-oligomeric γ-aminobutyric acid (GABA)-gated chloride channel of Drosophila melanogaster expressed in Xenopus oocytes. Sensitivity to the block by KN244 of the response to 30 μM GABA (IC50=41.6 nM, wild-type RDLac) was reduced abut 100 fold (IC50=4.5 μM) in the dieldrin-resistant (RDLacA302S) form of RDL.
Keywords: GABA-gated Cl− channel, KN244 (a tricyclic dinitrile), Drosophila melanogaster, RDL (resistant to dieldrin) subunit
Introduction
Ionotropic GABA receptors mediate fast, mostly inhibitory synaptic transmission in the nervous systems of vertebrates and invertebrates (Sattelle, 1990). Insect GABA gated-chloride channels are targets of several classes of chemicals with insecticidal activity including dieldrin, picrotoxinin (PTX) and BIDN (3, 3-bis(trifluoromethyl)-bicyclo [2,2,1]-heptane-2, 2-dicarbonitrile) (Figure 1a) (Hosie et al., 1997). However, the potency of these compounds is profoundly reduced in certain mutant Drosophila GABA receptor subunits encoded by the Rdl (resistant to dieldrin) gene (ffrench-Constant et al., 1991; 1993) which exhibit a serine to alanine substitution (RDLacA302S) in the second transmembrane region of Rdl-encoded subunits.
Figure 1.
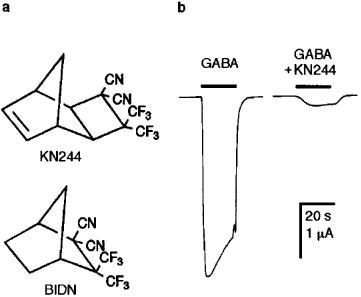
(a) Chemical structures of KN244 and its parent compound BIDN. (b) Actions of KN244 on the response of expressed Drosophila wild-type receptors (RDL homo-oligomers) to 30 μM GABA. Control response to GABA is shown (left) alongside the response to the same concentration of GABA following a 2 min application of 2.7 μM KN244 (right).
A BIDN analogue, (1α, 2β, 5β, 6α)-4, 4-bis(trifluoromethyl)-tricyclo [4, 2, 1, 02,5] non-7-ene-3, 3-dicarbonitrile, referred to as KN244 (Figure 1a), with an added double bond and a tricyclic structure has been tested on wild-type RDLac and dieldrin-resistant (RDLacA302S) homo-oligomeric Drosophila GABA-gated chloride channels expressed in Xenopus oocytes. KN244 is a potent antagonist of the wild-type receptor and shows cross-resistance to dieldrin in the RDLacA302S mutant.
Methods
Procedures for cloning, subcloning, synthesis of cRNA encoding the wild-type (RDLac) and mutant (RDLacA302S) forms of one of the four splice variants of RDL and preparation of oocytes have been described earlier (ffrench-Constant et al., 1991; Hosie et al., 1995a). Electrophysiological experiments were performed in a 90 μl chamber in which oocytes were perfused continuously with saline (5 ml min−1). Membrane currents were monitored under two-electrode voltage-clamp at −60 mV using 2 M KCl-filled electrodes (0.5–5 MΩ) and a GENECLAMP 500 amplifier (Axon Instruments, U.S.A.). Signals were displayed on an oscilloscope (Nicolet, U.S.A.) and stored on computer using Axotape software (Axon Instruments, U.S.A.). In all experiments, GABA was bath-applied for 20 s, and 5 min was allowed between each challenge to ensure stability of the GABA responses. KN244 was prepared by the [2π+2σ+2σ] cycloadditions of quadricyclane (quadricyclo[2.2.1.02,6.03.5] heptane) with a dienophile (Smith, 1966; Tabushi et al., 1972). KN244 was applied for 2 min prior to co-application of GABA and KN244. The peak amplitude of each GABA response was measured. Numerical data are shown as the mean±s.e.mean of at least three separate experiments. Dose-response data were analysed by means of GraphPad Prism (GraphPad Software, U.K.) using logistic equations (1) and (2) (c.f. Hosie et al., 1995a):
where Imax and Imin are amplitudes of the maximum and the minimum currents, respectively, and nH is the Hill coefficient.
All reagents for RNA synthesis were purchased from Promega (U.K.) except for m7G(5′)PPP(5′)G cap analogue, which was obtained from NEB (U.K.). Collagenase type IA and GABA were obtained from Sigma (U.K.). Plasmids coding wild-type and mutant RDLac were gifts from Dr R.T. Roush, Cornell University (U.S.A.).
Results
The tricyclic dinitrile, KN244 (Figure 1a), at concentrations of 10 nM and above, suppressed the amplitude of GABA-gated currents recorded from oocytes expressing RDLac homo-oligomers (Figure 1b). The EC50 for GABA was shifted from 28 μM (95% confidence interval (CI)=25–31 μM) to 171 μM (CI=161–183 μM) in the presence of 2.7 μM KN244 (Figure 2a) and the estimated Hill coefficients in the absence and presence of KN244 were both 1.7±0.1. The compound also reduced the amplitude of the maximum response to GABA to 91.4±0.8% of the control (Figure 2a).
Figure 2.
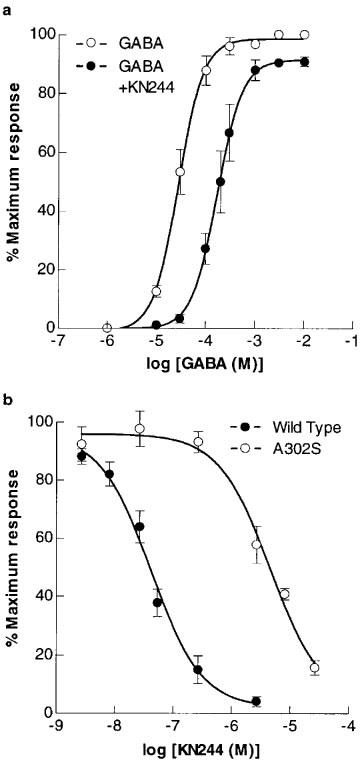
Blocking actions of KN244 on the response of expressed RDL homo-oligomers to GABA. (a) Dose-response curves for GABA on wild-type RDL receptors are shown in the absence and presence of KN244 (2.7 μM). (b) Actions of KN244 on the responses to 30 μM GABA of wild-type and mutant homo-oligomers. Each point plotted is the mean of 3–6 observations, and vertical bars represent±one standard error of the mean.
KN244 inhibited the response of both wild-type and mutant homo-oligomers to 30 μM GABA, a concentration close to the EC50 for GABA. However, whereas the IC50 for KN244 on the wild-type RDLac receptor was 41.6 nM (CI=20.1–85.9 nM) (Figure 2b), it was 4.5 μM (CI=1.9–10.8 μM) on the dieldrin-resistant form (RDLacA302S). No use-dependence in KN244 blockade was observed following a 2 min preincubation in the antagonist. Thus the IC50 for the response amplitude measured 15 s after the onset of the GABA response of RDLac was 33.8 nM (CI=19.2–59.3 nM), not markedly different from that at the onset of the response.
Discussion
This study of heterologously expressed D. melanogaster RDLac homomers describes for the first time the actions of KN244, a novel tricyclic dinitrile. Whereas the related compound BIDN is more effective in displacing radiolabelled convulsants from insect compared to rat brain membranes, the reverse is true for KN244 (Rauh et al., 1997). Here we have shown that the tricyclic dinitrile lowers the affinity of the receptor for GABA and reduces the maximum amplitude of the GABA response (Figure 1), suggesting that KN244 antagonism is neither purely competitive, nor entirely non-competitive, consistent with an allosteric coupling between agonist and antagonist binding sites. Such mixed antagonism, has been described for PTX on RDLac (Shirai et al., 1995), and on crustacean muscle (Smart & Constanti, 1986), as well as for BIDN (Hosie et al., 1995b) and fipronil (Hosie et al., 1995a) on RDLac. The potencies of all these antagonists are affected by the A302S mutation in a channel-lining residue of the second transmembrane region, suggesting that they either recognize common features of the GABA receptor, or share a common mechanism of action. Thus KN244 is the most potent convulsant tested to date on RDLac and offers a useful probe for the GABA receptor convulsant site of insects and vertebrates.
Abbreviations
- BIDN
3, 3-bis(trifluoromethyl)-bicyclo[2,2,1]-heptane-2, 2-dicarbonitrile
- PTX
picrotoxinin
References
- FFRENCH-CONSTANT R.H., MORTLOCK D.P., SCHAFFER C.D., MACINTYRE R.J., ROUSH R.T. Molecular cloning and transformation of cyclodiene resistance in Drosophila: an invertebrate γ-aminobutyric acid subtype A receptor locus. Proc. Natl. Acad. Sci. U.S.A. 1991;88:7209–7213. doi: 10.1073/pnas.88.16.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FFRENCH-CONSTANT R.H., ROCHELEAU T.A., STEICHEN J.C., CHALMERS A.E. A point mutation in a Drosophila GABA receptor confers insecticide resistance. Nature. 1993;363:449–451. doi: 10.1038/363449a0. [DOI] [PubMed] [Google Scholar]
- HOSIE A.M., ARONSTEIN K., SATTELLE D.B., FFRENCH-CONSTANT R.T. Molecular biology of insect neuronal GABA receptors. Trends Neurosci. 1997;20:578–583. doi: 10.1016/s0166-2236(97)01127-2. [DOI] [PubMed] [Google Scholar]
- HOSIE A.M., BAYLIS H.A., BUCKINGHAM S.D., SATELLE D.B. Actions of the insecticide fipronil, on dieldrin-sensitive and -resistant GABA receptors of Drosophila melanogaster. Br. J. Pharmacol. 1995a;115:909–912. doi: 10.1111/j.1476-5381.1995.tb15896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOSIE A.M., SHIRAI Y., BUCKINHAM S.D., RAUH J.J., ROUSH R.T., BAYLIS H.A., SATTELLE D.B. Blocking actions of BIDN, a bicyclic dinitrile convulsant compound, on wild-type and dieldrin-resistant GABA receptor homo-oligomers of Drosophila melanogaster expressed in Xenopus oocytes. Brain Res. 1995b;693:257–260. doi: 10.1016/0006-8993(95)00605-p. [DOI] [PubMed] [Google Scholar]
- RAUH J.J., HOLYOKE C.W., KLEIER D.A., PRESNAIL J.K., BENNER E.A., CORDOVA D., HOWARD M.H., HOSIE A.M., BUCKINGHAM S.D., BAYLIS H.A., SATTELLE B.D. Polycyclic dinitriles: a novel class of potent GABAergic insecticides provides a new radioligand, [3H]BIDN. Invertebrate Neurosci. 1997;3:261–268. doi: 10.1007/BF02480383. [DOI] [PubMed] [Google Scholar]
- SATTELLE D.B. GABA receptors of insect. Adv. Insect Physiol. 1990;22:1–113. [Google Scholar]
- SHIRAI Y., HOSIE A.M., BUCKINGHAM S.D., HOLYOKE C.W. , JR, BAYLIS H.A., SATTELLE D.B. Actions of picrotoxinin analogues on an expressed, homo-oligomeric GABA receptor of Drosophila melanogaster. Neurosci. Lett. 1995;189:1–4. doi: 10.1016/0304-3940(95)11432-v. [DOI] [PubMed] [Google Scholar]
- SMART T.G., CONSTANTI A. Studies on the mechanism of action of picrotoxinin and other convulsants at the crustacean muscle GABA receptor. Proc. R. Soc. B. 1986;227:191–216. doi: 10.1098/rspb.1986.0019. [DOI] [PubMed] [Google Scholar]
- SMITH C.D. Cycloaddition reactions of ‘Quadricyclanes'. J. Am. Chem. Soc. 1966;88:4273–4274. [Google Scholar]
- TABUSCHI I., YAMAMURA K., YOSHIDA Z.-I. Regio- and stereospecific [2π+2σ+2σ] cycloaddition reaction of quadricyclane. J. Am. Chem. Soc. 1972;94:787–792. [Google Scholar]


