Abstract
Adenosine 5′-diphosphate (ADP) induces human blood platelets to aggregate and change shape, and it has been suggested that these two responses are mediated by more than one subtype of ADP receptor.
The structure-activity relationships for several analogues of adenine nucleotides in causing aggregation and shape change were measured and compared in washed platelets using an aggregometer. ADP and its analogues 2-methylthioadenosine 5′-diphosphate (2-methylthio-ADP), adenosine 5′(α,β-methylene)diphosphonate (AMPCP), SP-adenosine 5′-O-(1-thiodiphosphate) (AD-PαS) and adenosine 5′-O-(2-thiodiphosphate) (ADPβS) were used as agonists. Adenosine 5′-triphosphate (ATP) and its analogues, P1, P5-diadenosine pentaphosphate (Ap5A), adenosine (5′-(α,β-methylene)triphosphonate (AMPCPP), 2-methylthioadenosine 5′-triphosphate (2-methylthio-ATP) and uridine 5′-triphosphate (UTP), as well as the trypanocidal drug suramin, were used as antagonists.
In general, the structure-activity relationships for both responses were similar, but for some analogues differences were observed. ADPαS and ADPβS were much more potent agonists relative to ADP for shape change than for aggregation and indeed ADPαS antagonized ADP-induced aggregation with an apparent pKB value of 5.5±0.1. 2-Methylthio-ATP also had different effects in aggregation and shape change, being a much higher affinity antagonist of aggregation than of shape change with an apparent pKB value of 7.0±0.2 for aggregation and 5.2±0.2 for shape change.
These results support the suggestion that these two responses are mediated by multiple ADP receptors on human platelets, and are consistent with shape change being mediated via one receptor (the P2Y1 receptor) with aggregation requiring the activation of two receptors (the P2Y1 and another P2Y receptor).
Keywords: Human platelets, ADP, ATP, P2 receptors, aggregation, shape change
Introduction
In human platelets adenosine 5′-diphosphate (ADP) causes aggregation, shape change, increase in cytosolic calcium concentration and inhibition of stimulated adenylate cyclase. Although all these responses are stimulated by ADP, whether these multiple effects of ADP on platelets can be explained by interaction with a single receptor subtype has been for many years the subject of controversy and a number of models have been proposed which include two or more ADP receptors (see e.g., Colman, 1992; Hourani & Hall, 1994; 1996; Mills, 1996; Gachet et al., 1996; Hourani et al., 1998; Kunapuli, 1998). Recently however two families of nucleotide receptors (P2 receptors) have been cloned, the P2Y (G protein-coupled) and P2X (ion channel) types, and of these the P2Y1 and P2X1 have been shown to be present on platelets, with the P2Y1 being involved in aggregation as discussed below while the P2X1 subtype, although shown to be involved in ADP-induced calcium entry, does not appear to play an important functional role (Léon et al., 1997; Vial et al., 1997). Some adenine nucleotide derivatives including adenosine 3′-phosphate 5′-phosphosulphate (A3P5PS) have been reported to act as specific P2Y1 antagonists (Boyer et al., 1996) and this compound has been shown by a number of groups to inhibit competitively the effects of ADP in causing aggregation, shape change and intracellular Ca2+ mobilization, suggesting that these responses, but not the ability of ADP to inhibit adenylate cyclase, are mediated wholly or in part via the P2Y1 receptor (Hechler et al., 1998a; Savi et al., 1998; Jin & Kunapuli, 1998; Jin et al., 1998; Park et al., 1998). This suggests the existence of another platelet ADP receptor, tentatively called P2YAC (sometimes P2T or P2YADP), coupled to Gi-mediated inhibiton of adenylate cyclase. The 2-alkylthio-substituted analogues 2-methylthioadenosine 5′-(β,γ-methylene)triphosphonate and 2-ethylthioadenosine 5′-monophosphate both competitively inhibit the effect of ADP on adenylate cyclase but only partially inhibit aggregation, suggesting that there are two components to aggregation, only one of which is associated with inhibition of adenylate cyclase (Hourani et al., 1986; 1996). The structurally-related compound 2-propylthioadenosine 5′-(β,γ-difluoromethene)triphosphonate (ARL66096), a potent inhibitor of ADP-induced platelet aggregation (Humphries et al., 1995), also blocks ADP-induced inhibition of adenylate cyclase, but does not inhibit ADP-mediated intracellular Ca2+ mobilization, inositol trisphosphate formation, or shape change (Sanderson et al., 1996; Daniel et al., 1998). A model has been proposed in which there are two separate G protein-coupled receptors for ADP on platelets (in addition to the P2X1 receptor), one (P2YAC) coupled to inhibition of adenylate cyclase and the other (P2Y1) responsible for shape change and coupled to phospholipase C and Ca2+ mobilization, and that coactivation of these two receptors is essential for full expression of ADP-induced platelet aggregation (Jin et al., 1998; Jin & Kunapuli, 1998; Kunapuli, 1998; Hechler et al., 1998a).
Various adenine nucleotide derivatives have been tested for their effects on ADP induced aggregation, intracellular Ca2+ mobilization and inhibition of stimulated adenylate cyclase activity in human blood platelets (Cusack & Hourani, 1981a,1981b; 1982a,1982b; Hall et al., 1994; Hall & Hourani, 1993; 1994; Hourani et al., 1992; Hourani & Hall, 1996; Geiger et al., 1998). However, the effects of these derivatives on shape change in comparison to the other responses of platelets have not been quantitatively investigated. In the present study we therefore investigated the effects of a range of nucleotides on the two functional responses, aggregation and shape change, in human washed platelets and compared them to allow further characterization of the platelet ADP receptors mediating these responses. Some of these results have been previously reported in abstract form (Park & Hourani, 1998; 1999).
Methods
Preparation of washed platelets
Venous blood from healthy human volunteers, who had not taken any drugs for the previous 10 days, was drawn into one sixth of the volume of acid-citrate-dextrose anticoagulant (25 g l−1 tri-sodium citrate dihydrate, 15 g l−1 citric acid monohydrate, 20 g l−1 glucose) and centrifuged at 260×g for 20 min. The supernatant platelet-rich plasma was removed and centrifuged at 680×g for 20 min in the presence of prostacyclin (1 μM) to precipitate the platelets. The supernatant plasma was discarded and the platelets were suspended in 10 ml of HEPES-saline (NaCl 145 mM, KCl 5 mM, glucose 10 mM, N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulphonic acid] (HEPES) 10 mM, 2 mg ml−1 bovine serum albumin adjusted to pH 7.4 with 1 M NaOH). The platelets were again collected by centrifugation at 680×g for 20 min in the presence of prostacyclin (1 μM) and resuspended at 2×108 ml−1 in HEPES-saline.
Aggregation and shape change
250 μl aliquots of washed platelets were taken into siliconized glass aggregometer tubes and incubated at 37°C for 3 min prior to transfer to a Chronolog lumi-aggregometer (Havertown, U.S.A.). The platelet suspension was maintained at 37°C within the aggregometer and stirred at 1000 r.p.m. Agonists were added in 10 μl of water and antagonists, when used, were added simultaneously with the agonist to avoid possible complicating effects of pharmacologically active degradation products. Calcium (CaCl2) was added to the platelets at a final concentration of 1 mM 3 min before addition of agonist, and for aggregation experiments fibrinogen was added to the platelets 10 s before agonist at a final concentration of 0.3 mg ml−1. Aggregation was quantified as the maximal rate of increase in light transmission (in arbitrary units per min) through the stirred sample relative to HEPES-saline, on addition of agonist. To quantify shape change, the maximal decrease (in arbitrary units) in light transmission through the stirred sample at 37°C on addition of the agonist was measured. In the absence of fibrinogen aggregation in response to ADP was negligible as measured in the aggregometer. Aggregation and shape change experiments were both carried out in parallel, usually in triplicate, on platelets from each donor, and log concentration-response curves were obtained on platelets obtained from at least three donors.
Measurement of adenylate cyclase activity
Adenylate cyclase activity was measured by the method of Haslam & Rosson (1975) as described in detail in Hall & Hourani (1994) by measuring levels of [14C]-adenosine 3′,5′-cyclic monophosphate (cyclic AMP) present after stimulation of platelets preincubated in plasma for 45 min at 37°C with [14C]-adenine. The labelled platelets were washed as before and resuspended in HEPES buffer, and aliquots (450 μl) were taken and preincubated for 3 min with CaCl2 (1 mM). Adenylate cyclase was stimulated for 30 s at 37°C by addition of prostaglandin E1 (PGE1) (1 μM) in the presence of the phosphodiesterase inhibitor isobutylmethylxanthine (IBMX) (0.1 mM) and in the presence or absence of ADP and/or 2-methylthioadenosine 5′-triphosphate (2-methylthio-ATP), all added simultaneously in a volume of 50 μl. The adenylate cyclase activity was expressed as d.p.m. derived from [14C]-cyclic AMP in the samples, corrected for recovery during the extraction procedure.
Data analysis
All values were expressed as mean±s.e.mean. Agonist concentrations giving 50% maximal response were determined from the midpoint of lines drawn through the linear parts of the individual log concentration-response curves and expressed as the negative log of the concentration (p[A]50). For antagonist studies the concentration-ratios were calculated from the shift in the [A]50 values, and the inhibitor concentration divided by (concentration-ratio-1) was taken as the apparent dissociation constant of the inhibitor and expressed as a negative logarithm (pKB). All lines were drawn by linear regression analysis. The significance of differences between mean p[A]50 or pKB values for the two effects was assessed by unpaired Student's t-test, and P<0.05 was considered statistically significant.
Drugs
ADP, adenosine 5′-(α,β-methylene)diphosphonate (AMPCP), ATP, P1,P5-diadenosine pentaphosphate (Ap5A), adenosine 5′-(α,β-methylene)triphosphonate (AMPCPP), uridine 5′-triphosphate (UTP), bovine serum albumin, prostacyclin, and diethlaminoethyl (DEAE)-Sephadex A-25 were obtained from Sigma Chemicals Company (Poole, U.K.). Adenosine 5′-O-(2-thiodiphosphate) (ADPβS) was obtained from Boehringer Mannheim (Germany) and Sp-adenosine 5′-O-(1-thiotriphosphate) (ADPαS) was obtained from BIOLOG Life Science Institute (Bremen, Germany). 2-Methylthio-ATP and 2-methylthioadenosine 5′-diphosphate (2-methylthio-ADP) were obtained from Research Biochemicals Incorporated (Poole, U.K.). Suramin was a generous gift from Bayer, U.K. Most of the analogues were used as they were purchased, except for 2-methylthio-ATP, which contained about 20% 2-methylthio-ADP as an impurity when it was analysed by high pressure liquid chromatography. It was therefore purified before use by ion-exchange chromatography on DEAE-Sephadex eluted by a linear gradient of 1–800 mM triethylammonium bicarbonate.
Results
ADP concentration-dependently induced aggregation and shape change and had a slightly higher potency for shape change (Figures 1, 2, 3, 4, 5 and 6; Table 1). 2-Methylthio-ADP also induced aggregation and shape change with a similar potency, and was approximately ten times more potent than ADP for each effect (Figure 1a and b). AMPCP appeared to be a low efficacy partial agonist for aggregation, causing a response at 1 μM but achieving less than 25% of the response to ADP at 100 μM (Figure 1c). No agonist effect was observed for shape change at concentrations of AMPCP up to 100 μM (Figure 1d). ADPβS was a much more potent agonist for shape change than for aggregation (Figure 2). It caused shape change with 8 fold less potency than ADP, but was almost inactive in causing aggregation, achieving at 100 μM only 26% of the maximum aggregation induced by ADP. ADPαS also showed differential effects on the responses, being almost as potent as ADP for shape change (Figure 3c), whereas it was almost inactive in causing aggregation (Figure 3a). Log concentration-response curves to ADP in the presence and absence of ADPαS (10 μM) showed that ADPαS antagonized the effect of ADP as an aggregating agent in an apparently competitive manner with an apparent pKB of 5.4±0.1 (Figure 3b). Overall, the potency order of agonists was 2-methylthio-ADP≫ADP≫AMPCP, ADPβS for aggregation with ADPαS being an antagonist, and 2-methylthio-ADP≫ADP>ADPαS>ADPβS≫AMPCP for shape change (see Table 1).
Figure 1.
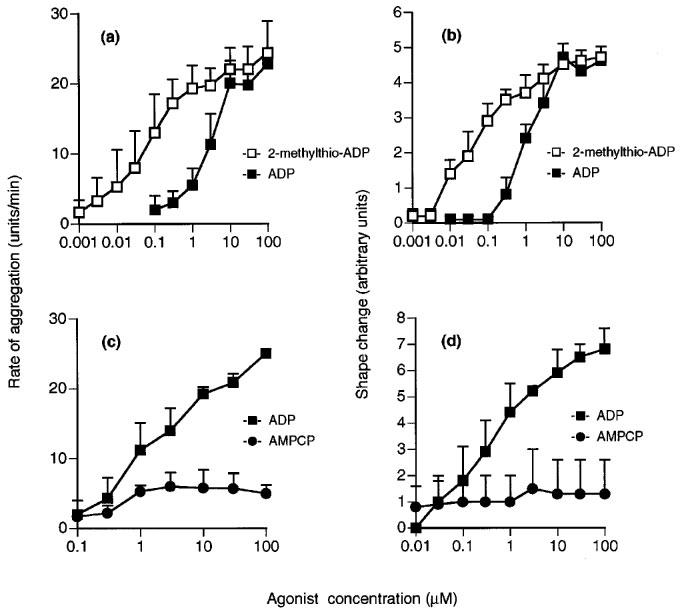
Effects of ADP (a–d), 2-methylthio-ADP (a and b) or AMPCP (c and d) on aggregation (a and c) and shape change (b and d) of human washed platelets. Each point is the mean of at least three determinations and vertical bars show the s.e.mean. For abbreviations, see text.
Figure 2.
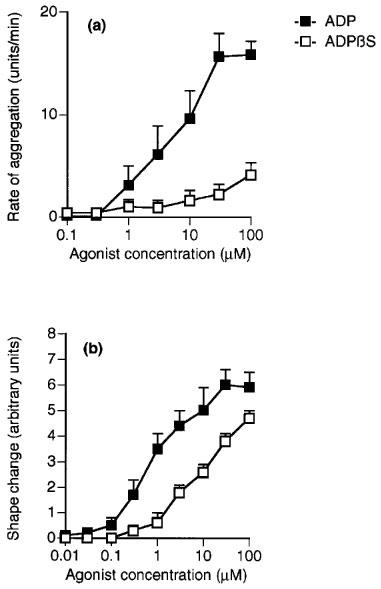
Effects of ADPβS and ADP on (a) aggregation and (b) shape change of human washed platelets. Each point is the mean of at least three determinations and the vertical bars show the s.e.mean. For abbreviations, see text.
Figure 3.
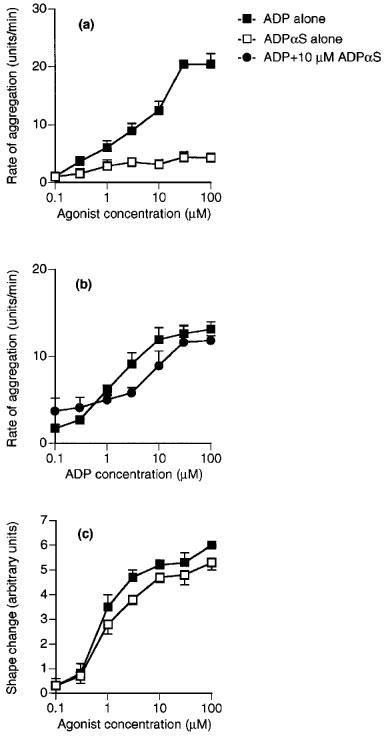
Effects of ADPαS and ADP on (a) aggregation and (c) shape change of human washed platelets. (b) Aggregation of washed platelets induced by ADP alone or in the presence of 10 μM ADPαS. Each point is the mean of at least three determinations and the vertical bars show the s.e.mean. For abbreviations, see text.
Figure 4.
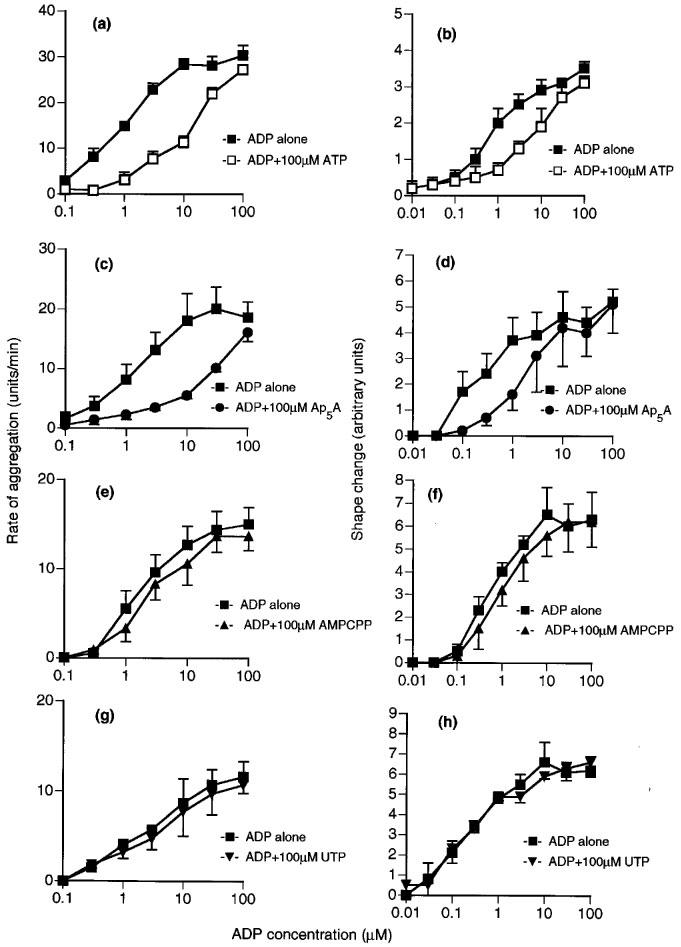
Effects of ATP analogues on ADP-induced aggregation (a, c, e and g) and shape change (b, d, f and h) of human washed platelets. Responses to ADP are shown alone or in the presence of 100 μM ATP (a and b), Ap5A (c and d), AMPCPP (e and f) or UTP (g and h). Each point is the mean of at least three determinations and the vertical bars show the s.e.mean. For abbreviations, see text.
Figure 5.
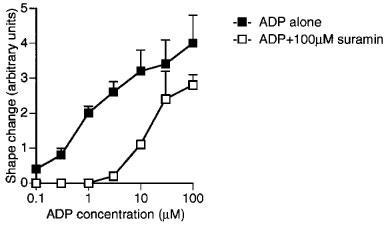
Effects of suramin (100 μM) on ADP-induced shape change of human washed platelets. Each point is the mean of at least three determinations and the vertical bars show s.e.mean. For abbreviations, see text.
Figure 6.
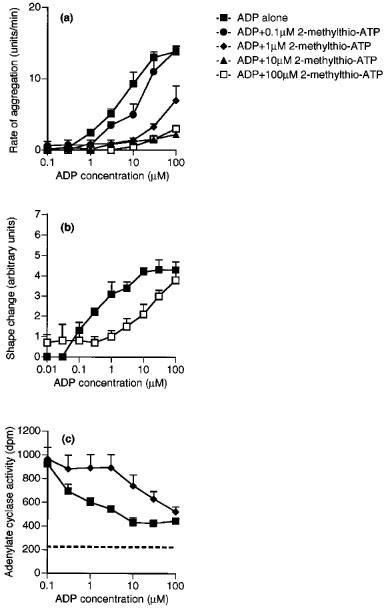
Effects of 2-methylthio-ATP on ADP-induced aggregation (a), shape change (b) and adenylate cyclase activity (c) of washed platelets. Responses to ADP are shown alone or in the presence of 0.1, 1, 10 or 100 μM 2-methylthio-ATP. The dotted line on (c) shows the adenylate cyclase activity in the presence of IBMX (0.1 mM) alone. Each point is the mean of at least three determinations, and the vertical bars show the s.e.mean. For abbreviations, see text.
Table 1.
p[A]50 values for agonists and apparent pKB values for antagonists for ADP-induced aggregation and shape change of human washed platelets
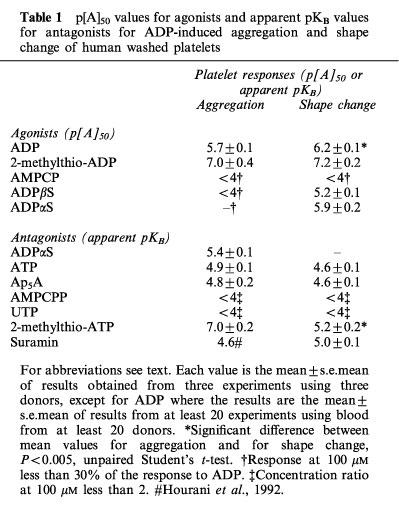
Both responses of ADP were inhibited by ATP (100 μM), which caused parallel shifts to the right of the log concentration-response curve to ADP to a similar extent for both responses (Figure 4a and b). The effects of ADP on aggregation and shape change were also inhibited to a similar extent in an apparently competitive manner by Ap5A (100 μM) (Figure 4c and d), but little inhibition was observed either for aggregation or for shape change by AMPCPP (100 μM) (Figure 4e and f) or UTP (100 μM) (Figure 4g and h). Suramin (100 μM), which has been reported to be a non-selective P2 antagonist, acted as an antagonist for shape change, having an apparent pKB value of 5.0±0.1 (Figure 5), which is similar to the previously reported pKB for aggregation of 4.6 (Hourani et al., 1992). 2-Methylthio-ATP was a much higher affinity antagonist of aggregation than of shape change, being inactive as an antagonist for shape change at 1 or 10 μM (results not shown) while at 100 μM it caused a parallel shift to the right of the concentration-response curve with an apparent pKB of 5.2±0.2 (Figure 6b). However, 2-methylthio-ATP (10 or 100 μM) almost abolished the aggregation induced by ADP (Figure 6a), and an apparent pKB value of 7.0±7.2 was calculated for this effect, which is highly significantly different from the apparent pKB value for inhibition of shape change. In addition 2-methylthio-ATP (1 μM) antagonized the inhibition by ADP of PGE1-stimulated adenylate cyclase in an apparently competitive manner with an apparent pKB value of 7.2±0.2, which is not significantly different from the apparent pKB value for inhibition of aggregation (Figure 6c). Overall, the apparent affinity order for antagonists was 2-methylthio-ATP≈ >ADPαS 4ATP=Ap5A⩾ suramin>AMPCPP,UTP for aggregation, and 2-methylthio-ATP⩾suramin>ATP=Ap5A>AMPCPP UTP for shape change (Table 1).
Discussion
These results represent the first detailed comparison of the structure-activity relationships of a group of nucleotide analogues at the human platelet ADP receptors mediating aggregation and shape change in platelets. The effects of various adenine nucleotide analogues on aggregation, increases in calcium and inhibition of adenylate cyclase have already been tested, but it is important to compare directly the structure-activity relationships for shape change and for aggregation since it is still not clear whether both these functional responses are mediated by one common ADP receptor. Also, in our previous studies aggregation was measured in plasma, but in the current experiments all the conditions (including the calcium concentration) were kept the same for the aggregation and the shape change measurements, which were carried out in parallel on the same platelet preparations in buffer. The fact that the calcium concentration was the same is particularly important as this has been shown to affect both ADP-induced shape change and the effect of ADP on adenylate cyclase (Hall et al., 1994). The only difference in the studies presented here was the presence and absence of fibrinogen which was necessary for aggregation but not for shape change. It should be noted however that for most of the antagonists only a single concentration was tested so only an apparent pKB could be calculated, and that the antagonists were not preincubated with the platelets (to avoid breakdown) and the system was unlikely to be at equilibrium. In addition, the measure of aggregation used was the initial rate of aggregation and there is some evidence that different conclusions can be drawn when using different measures, at least in plasma (Jarvis et al., 1998), although as discussed below the situation may be different in washed platelets.
Among the agonists we tested, the phosphorothioate analogues of ADP, ADPαS and ADPβS, showed different effects for aggregation and shape change. ADPβS caused shape change with a much higher potency relative to ADP than aggregation while ADPαS showed an even greater difference, being as potent as ADP for shape change but acting as an antagonist for ADP-induced aggregation. The extent of aggregation caused by these two analogues were much lower in washed platelets than previously reported in platelets in plasma, where they were both partial agonists achieving approximately 50% of the maximal response to ADP (Cusack & Hourani, 1981a,1981b). This either suggests that some unknown factors are lost from the plasma when they are washed, or that different conditions (e.g., calcium concentrations) affect the responses. Alternatively, the fact of washing the platelets may selectively desensitize some components of the aggregation process. ADPβS has also been shown to act as a partial agonist for inhibition of PGE1-stimulated adenylate cyclase and for increases in cytosolic calcium concentration (Cusack & Hourani, 1981a; Hall & Hourani, 1993). However, ADPαS acts as an antagonist for inhibition of PGE1-stimulated adenylate cyclase, whilst acting as a partial agonist for increases in cytosolic calcium concentration (Cusack & Hourani, 1981b; Hall & Hourani, 1993). The results presented here demonstrate clearly that the two compounds exhibit different effects with regard to shape change and aggregation, supporting the idea that these two responses of platelets are mediated by multiple ADP receptors. In the case of ADPαS the results can be explained in terms of the two-receptor model with ADPαS being an antagonist at the P2YAC receptor but an agonist at the P2Y1 receptor, as its apparent KB value calculated here for inhibition of aggregation (5.4) is very similar to that previously reported for its antagonism of the effect of ADP on adenylate cyclase (5.13; Cusack & Hourani, 1981b).
Of the agents tested as antagonists, ATP, Ap5A and suramin showed similar apparent affinities as antagonists for ADP-induced aggregation and shape change, causing the log concentration-response curve to ADP to shift to the right in a parallel fashion. AMPCPP and UTP were essentially inactive as inhibitors for both aggregation and shape change. We and others have shown that P2Y1 antagonists such as A3P5PS also inhibit both responses in a similar manner (Park et al., 1998; Hechler et al., 1998b; Savi et al., 1998; Jin et al., 1998). Indeed in our experiments, carried out using an identical protocol to the experiments described here, A3P5PS (100 μM) had apparent pKB values of 5.2 and 5.8 as an antagonist of ADP-induced aggregation and shape change respectively (Park et al., 1998). However, like ADPβS and ADPαS, 2-methylthio-ATP is another compound which can pharmacologically discriminate aggregation and shape change, showing much higher affinity antagonism of ADP-induced aggregation than of shape change. Similar discrimination has been observed for the structurally related analogue ARL66096 (Sanderson et al., 1996). In addition, 2-methylthio-ATP (like the related analogues ARL66096, 2-methylthioadenosine 5′-(β,γ-methylenetriphosphonate) and 2-ethylthioadenosine 5′-monophosphate (Hourani et al., 1986; 1996; Daniel et al., 1998; Jin et al., 1998)) inhibited the effect of ADP on adenylate cyclase activity in an apparently competitive manner, and its apparent pKB for this (7.2) is very similar to its apparent pKB value for inhibition of aggregation (7.0). 2-Methylthio-ATP (50 μM) also inhibited ADP-induced increases in cytosolic calcium concentration and an apparent pKB value of around 5.3 (similar to its apparent pKB value of 5.2 for inhibition of shape change) can be calculated from the results reported, although some depression of the maximal response to ADP was observed (Hall & Hourani, 1993). These values are similar to the pKi value of 5.2 reported for inhibition of calcium mobilization in Jurkat cells transfected with the human P2Y1 receptor (Hechler et al., 1998b). Again, these results are consistent with a two-receptor model for platelet aggregation, with 2-methylthio-ATP having a higher affinity for the P2YAC receptor than for the P2Y1 receptor. It should be noted however that the apparently competitive antagonist effect of 2-methylthio-ATP for aggregation seen here in washed platelets was different from the non-competitive inhibition previously seen in platelets in plasma, where a component of the response was clearly resistant to inhibition (Cusack & Hourani, 1982c).
According to the present study, ADP-induced responses of washed platelets appear to be well explained by a model involving two G protein-coupled receptors, with one (P2Y1) coupled to phospholipase C via Gq and responsible for shape change, and the other (P2YAC) coupled to G1 mediating inhibition of adenylate cyclase, with both receptors being required to be activated for aggregation, at least in washed platelets (Jin et al., 1998; Jin & Kunapuli, 1998; Kunapuli, 1998; Hechler et al., 1998a). In this model 2-methylthio-ATP has a higher affinity for P2YAC than P2Y1, ADPβS has a higher potency at P2Y1 than P2YAC, and ADPαS acts as an agonist at the P2Y1 but an antagonist at P2YAC. If both receptors need to be activated simultaneously to cause aggregation, then for any agonists which have a different potency for P2Y1 and P2YAC, the extent of aggregation will be determined by the lower potency receptor. It should be noted however that the relative potency of ADPβS compared to ADP obtained here for shape change is rather lower than that previously reported for the turkey P2Y1 receptor where ADPβS has been reported to be approximately twice as potent as ADP as an activator of phospholipase C (Filtz et al., 1994). For antagonists, however, binding to only one receptor, P2Y1 or P2YAC, is enough to inhibit aggregation, so the pKB for inhibition of aggregation reflects the higher of the two pKB values.
However, although this model fits the responses in washed platelets, the responses of platelets in plasma are not identical. In plasma, ADPβS and ADPαS act as partial agonists for aggregation achieving a much higher maximal response compared to washed platelets (Cusack & Hourani, 1981a,1981b). Also in the case of 2-methylthio-ATP, ADP-induced aggregation was only inhibited by about 50% even at a high concentration of 2-methylthio-ATP (100 μM) (Cusack & Hourani, 1982c), which completely abolished ADP-induced aggregation in washed platelets. Similar differences in the extent of inhibition was reported for 2-methylthioadenosine 5′-(β,γ-methylenetriphosphonate) (Humphries et al., 1994). It is possible that the P2Y1 receptors of washed platelets are in some way desensitized compared to those of platelets in plasma so that while in plasma activation of P2Y1 alone can induce aggregation, in washed platelets aggregation is absolutely dependent on co-activation of both receptors. That the P2Y1 receptors on washed platelets may be at low density or poorly coupled has been suggested from a comparison of [Ca2+]i responses between bovine P2Y1 receptors transfected into Jurkat cells and human washed platelets (Fagura et al., 1998). It has been suggested that P2Y1 and P2YAC contribute to cause aggregation of platlets in different ways, with P2Y1 being involved in rapid transient aggregation while P2YAC mediates later sustained aggregation (Jarvis et al., 1998). This is more consistent with the results obtained with platelets in plasma, where stimulation of P2Y1 receptors alone appears to be able to induce a sub-maximal aggregation, with activation of P2YAC being required for full expression of aggregation.
In conclusion, the results presented here not only provide further evidence in favour of a two-receptor model of platelet activation, but also indicate important differences between washed platelets and platelets in plasma which can greatly affect the results obtained with agonists and antagonists.
Abbreviations
- ADP
adenosine 5′-diphosphate
- ADPαS
SP-adenosine 5′-O-(1-thiodiphosphate)
- ADPβS
adenosine 5′-O-(2-thiodiphosphate)
- AMPCP
adenosine 5′-(α,β-methylene)diphosphonate
- AMPCPP
adenosine 5′-(α,β-methylene)triphosphonate
- Ap5A
P1,P5-diadenosine pentaphosphate
- A3P5PS
adenosine 3′-phosphate 5′-phosphosulphate
- ARL66096
2-propylthioadenosine 5′-(β,γ-difluoromethylene)triphosphonate
- ATP
adenosine 5′-triphosphate
- cyclic AMP
adenosine 3′,5′-cyclic monophosphonate
- DEAE
diethylaminoethyl
- HEPES
N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulphonic acid]
- IBMX
isobutylmethylxanthine
- 2-methylthio-ADP
2-methylthioadenosine 5′-diphosphate
- 2-methylthio-ATP
2-methylthioadenosine 5′-triphosphate
- PGE1
prostaglandin E1
- UTP
uridine 5′-triphosphate
References
- BOYER J.L., ROMERO-AVILA T., SCHACHTER J.B., HARDEN T.K. Identification of competitive antagonists of the P2Y1 receptor. Mol. Pharmacol. 1996;50:1323–1329. [PubMed] [Google Scholar]
- COLMAN R.W. Platelet ADP receptors stimulating shape change and inhibiting adenylate cyclase. News Physiol. Sci. 1992;7:274–278. [Google Scholar]
- CUSACK N.J., HOURANI S.M.O. Partial agonist behaviour of adenosine 5′-O-(2-thiodiphosphate) on human platelets. Br. J. Pharmacol. 1981a;73:405–408. doi: 10.1111/j.1476-5381.1981.tb10436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUSACK N.J., HOURANI S.M.O. Effects of Rp and Sp diastereoisomers of adenosine 5′-O-(1-thiodiphosphate) on human platelets. Br. J. Pharmacol. 1981b;73:409–412. doi: 10.1111/j.1476-5381.1981.tb10437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUSACK N.J., HOURANI S.M.O. Adenosine 5′-diphosphate antagonists and human platelets: no evidence that aggregation and inhibition of stimulated adenylate cyclase are mediated by different receptors. Br. J. Pharmacol. 1982a;76:221–227. doi: 10.1111/j.1476-5381.1982.tb09210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUSACK N.J., HOURANI S.M.O. Competitive inhibition by adenosine 5′-triphosphate of the actions on human platelets of 2-chloroadenosine 5′-diphosphate, 2-azidoadenosine 5′-diphosphate and 2-methylthioadenosine 5′-diphosphate. Br. J. Pharmacol. 1982b;77:329–333. doi: 10.1111/j.1476-5381.1982.tb09302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUSACK N.J., HOURANI S.M.O. Specific but non-competitive inhibition by 2-alkylthio analogues of adenosine 5′-monophosphate and adenosine 5′-triphosphate of human platelet aggregation induced by adenosine 5′-diphosphate. Br. J. Pharmacol. 1982c;75:397–400. doi: 10.1111/j.1476-5381.1982.tb08800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANIEL J.L., DANGELMAIER C., JIN J., ASHBY B., SMITH J.B., KUNAPULI S.P. Molecular basis for ADP-induced platelet activation. I. Evidence for three distinct ADP receptors on human platelets. J. Biol. Chem. 1998;273:2024–2029. doi: 10.1074/jbc.273.4.2024. [DOI] [PubMed] [Google Scholar]
- FAGURA M.S., DAINTY I.A., MCKAY G.D., KIRK I.P., HUMPHRIES R.G., ROBERTSON M.J., DOUGALL I.G., LEFF P. P2Y1-receptors in human platelets which are pharmacologically distinct from P2YADP-receptors. Br. J. Pharmacol. 1998;124:157–164. doi: 10.1038/sj.bjp.0701827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILTZ T.M., LI Q., BOYER J.L., NICHOLAS R.A., HARDEN T.K. Expression of a cloned P2Y purinergic receptor that couples to phospholipase C. Mol. Pharmacol. 1994;46:8–14. [PubMed] [Google Scholar]
- GACHET C., HECHLER B., LÉON C., VIAL C., OHLMANN P., CAZENAVE J.-P. Purinergic receptors on blood platelets. Platelets. 1996;7:261–267. doi: 10.3109/09537109609023587. [DOI] [PubMed] [Google Scholar]
- GEIGER J., HÖNIG-LIEDL P., SCHANZENBÄCHER P., WALTER U. Ligand specificity and ticlopidine effects distinguish three human platelet ADP receptors. Eur. J. Pharmacol. 1998;351:235–246. doi: 10.1016/s0014-2999(98)00305-7. [DOI] [PubMed] [Google Scholar]
- HALL D.A., FROST V., HOURANI S.M.O. Effects of extracellular divalent cations on responses of human blood platelets to adenosine 5′-diphosphate. Biochem. Pharmacol. 1994;48:1319–1326. doi: 10.1016/0006-2952(94)90553-3. [DOI] [PubMed] [Google Scholar]
- HALL D.A., HOURANI S.M.O. Effects of analogues of adenine nucleotides on increases in intracellular calcium mediated by P2T purinoceptors on human blood platelets. Br. J. Pharmacol. 1993;108:728–733. doi: 10.1111/j.1476-5381.1993.tb12869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL D.A., HOURANI S.M.O. Effects of suramin on increases in cytosolic calcium and on inhibition of adenylate cyclase induced by adenosine 5′-diphosphate in human platelets. Biochem. Pharmacol. 1994;47:1013–1018. doi: 10.1016/0006-2952(94)90412-x. [DOI] [PubMed] [Google Scholar]
- HASLAM R.J., ROSSON G.M. Effects of adenosine on levels of adenosine cyclic 3′,5′-monophosphate in human blood platelets in relation to adenosine incorporation and platelet aggregation. Mol. Pharmacol. 1975;11:528–544. [PubMed] [Google Scholar]
- HECHLER B., LÉON C., VIAL C., VIGNE P., FRELIN C., CAZENAVE J.-P., GACHET C. The P2Y1 receptor is necessary for adenosine 5′-diphosphate-induced platelet aggregation. Blood. 1998a;92:152–159. [PubMed] [Google Scholar]
- HECHLER B., VIGNE P., LÉON C., BREITTMAYER J.P., GACHET C., CAZENAVE J.-P. ATP derivatives are antagonists of the P2Y1 receptor: similarities to the platelet ADP receptor. Mol. Pharmacol. 1998b;53:727–733. [PubMed] [Google Scholar]
- HOURANI S.M.O., DIVIRGILIO F., LOUBATIÉRES-MARIANI M.-M.Physiological roles of P2 receptors in platelets, visceral smooth muscle and the immune and endocrine systems The P2 Nucleotide Receptors 1998Totowa NJ: Humana Press; 361–411.eds. Turner, J.T., Weisman, G.A. & Fedan, J.S. pp [Google Scholar]
- HOURANI S.M.O., HALL D.A. ADP receptors on human blood platelets. Trends Pharmacol. Sci. 1994;15:103–108. doi: 10.1016/0165-6147(94)90045-0. [DOI] [PubMed] [Google Scholar]
- HOURANI S.M.O., HALL D.A.P2T-Purinoceptors: ADP receptors on platelets P2 Purinoceptors: Localization, Function and Transduction Mechanisms. Ciba Foundation Symposium. 198 1996Chichester: Wiley; 53–70.eds. Chadwick, D.J. & Goode, J.A. pp [DOI] [PubMed] [Google Scholar]
- HOURANI S.M.O., HALL D.A, NIEMAN C.J. Effects of the P2-purinoceptor antagonist suramin on human platelet aggregation induced by adenosine 5′-diphosphate. Br. J. Pharmacol. 1992;105:453–457. doi: 10.1111/j.1476-5381.1992.tb14274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOURANI S.M.O., WELFORD L.A., CUSACK N.J. 2-MeS-AMP-PCP and human platelets: implications for the role of adenylate cyclase in ADP-induced aggregation. Br. J. Pharmacol. 1986;87:84P. [Google Scholar]
- HOURANI S.M.O., WELFORD L.A., CUSACK N.J. Effects of 2-methylthioadenosine 5′-β,γ-methylenetriphosphonate and 2-ethylthioadenosine 5′-monophosphate on human platelet activation induced by adenosine 5′-diphosphate. Drug Dev. Res. 1996;38:12–23. [Google Scholar]
- HUMPHRIES R.G., TOMLINSON W., INGALL A.H., CAGE P.A., LEFF P. FPL 66096: a novel, highly potent and selective antagonist at human platelet P2T-purinoceptors. Br. J. Pharmacol. 1994;113:1057–1063. doi: 10.1111/j.1476-5381.1994.tb17100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMPHRIES R.G., ROBERTSON M.J., LEFF P. A novel series of P2T purinoceptor antagonists: definition of the role of ADP in arterial thrombosis. Trends Pharmacol. Sci. 1995;16:179–181. doi: 10.1016/s0165-6147(00)89018-5. [DOI] [PubMed] [Google Scholar]
- JARVIS G.E., HUMPHRIES R.G., ROBERTSON M.J., LEFF P. ADP can induce aggregation of human platelets via both P2Y1 and P2T receptors. Br. J. Pharmacol. 1998;124:32P. doi: 10.1038/sj.bjp.0703046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIN J., DANIEL J.L., KUNAPULI S.P. Molecular basis for ADP-induced platelet activation. II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J. Biol. Chem. 1998;273:2030–2034. doi: 10.1074/jbc.273.4.2030. [DOI] [PubMed] [Google Scholar]
- JIN J., KUNAPULI S.P. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8070–8074. doi: 10.1073/pnas.95.14.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNAPULI S.P. Multiple P2 receptor subtypes on platelets: a new interpretion of their function. Trends Pharmacol. Sci. 1998;19:391–394. doi: 10.1016/s0165-6147(98)01248-6. [DOI] [PubMed] [Google Scholar]
- LÉON C., HECHLER B., VIAL C., LERAY C., CAZENAVE J.-P., GACHET C. The P2Y1 receptor is an ADP receptor antagonized by ATP and expressed in platelets and megakaryoblastic cells. FEBS Letts. 1997;403:26–30. doi: 10.1016/s0014-5793(97)00022-7. [DOI] [PubMed] [Google Scholar]
- MILLS D.C.B. ADP receptors on platelets. Thrombos. Haemostas. 1996;76:835–856. [PubMed] [Google Scholar]
- PARK H.-S., HOURANI S.M.O. Different effects of analogues of adenine nucleotides on aggregation and shape change mediated by adenosine 5′-diphosphate receptors on human platelets. Drug Dev. Res. 1998;43:48. [Google Scholar]
- PARK H.-S., HOURANI S.M.O. Different effects of adenine nucleotide analogues on the responses mediated by adenosine 5′ diphosphate (ADP) receptors on human platelets. Br. J. Pharmacol. 1999;126:196P. doi: 10.1038/sj.bjp.0702690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK H.-S., TENNANT J.P., WAKTOLLA G.F., SARKARDEI S., KASS G.E.N., HOURANI S.M.O. Effects of adenosine 3′-phosphate 5′-phosphosulphate on P2 receptors in platelets and smooth muscle preparations. Drug Dev. Res. 1998;45:67–73. [Google Scholar]
- SANDERSON H.M., HEPTINSTALL S., VICKERS J., LÖSCHE W. Studies on the effects of agonists and antagonists on platelet shape change and platelet aggregation in whole blood. Blood Coagulation Fibrinolysis. 1996;7:245–248. doi: 10.1097/00001721-199603000-00034. [DOI] [PubMed] [Google Scholar]
- SAVI P., BEAUVERGER P., LABOURET C., DELFAUD M., SALEL V., KAGHAD M., HERBERT J.M. Role of P2Y1 purinoceptor in ADP-induced platelet activation. FEBS Letts. 1998;422:291–295. doi: 10.1016/s0014-5793(98)00025-8. [DOI] [PubMed] [Google Scholar]
- VIAL C., HECHLER B., LÉON C., CAZENAVE J.-P. Presence of P2X1 purinoceptors in human platelets and megakaryoblastic cell lines. Thrombos. Haemostas. 1997;78:1500–1504. [PubMed] [Google Scholar]


