Abstract
The effects of endothelin (ET)-11–31 and ET-21–31, human chymase products of the corresponding big ETs, on the intracellular free Ca2+ concentration ([Ca2+]i) and [125I]-ET-1 binding were investigated using human cultured bronchial smooth muscle cells (BSMC).
ET-11–31 (10−8 M – 3×10−7 M) and ET-21–31 (3×10−8 M–3×10−6 M) caused an increase in [Ca2+]i in a concentration-dependent manner. Big ET-1 (3×10−8 M – 10−6 M) also caused this increase, but not big ET-2 at concentrations up to 10−6 M. The [Ca2+]i increase induced by ET-1 was inhibited by both BQ123, an ETA-receptor antagonist, and BQ788, an ETB-receptor antagonist, whereas that induced by ET-3 was inhibited by BQ788 but not by BQ123.
Increases in [Ca2+]i caused by ET-11–31, big ET-1 and ET-21–31 were completely inhibited by 10−4 M phosphoramidon, a dual neutral endopeptidase (NEP)/endothelin-converting enzyme (ECE) inhibitor, and 10−5 M thiorphan, a NEP inhibitor.
Scatchard plot analyses of the saturation curves of [125I]-ET-1 and [125I]-ET-3 showed that both ETA- and ETB- receptors at the ratio of 4 : 1 were expressed on BSMC. ET-11–31, big ET-1 and ET-21–31 inhibited [125I]-ET-1 binding in a concentration-dependent manner, and these effects were attenuated by treatment with thiorphan. On the other hand, big ET-2 slightly inhibited the binding at a high concentration and this was not affected by thiorphan.
These results suggest that ET-11–31, big ET-1 and ET-21–31 cause an increase in [Ca2+]i by being converted into the corresponding ET-1 and ET-2 by NEP, but this did not occur with big ET-2 in human BSMC. ET-21–31 produced by human chymase from big ET-2 might be important for the generation of ET-2 in human bronchial tissue.
Keywords: Endothelin, big endothelin, endothelin converting enzyme, neutral endopeptidase, chymase, bronchial smooth muscle cells
Introduction
Endothelin (ET), a highly potent vasoactive peptide containing 21 amino acid residues, exists as three different isoforms as ET-1, ET-2, and ET-3 (Inoue et al., 1989). All three isopeptides are generated from the corresponding big forms through a specific cleavage between Trp21 and Val22 (ET-1, ET-2) or Trp21 and Ile22 (ET-3). ET converting enzyme (ECE) is a membrane-bound neutral metalloprotease. Two distinct ECEs, termed ECE-1 and ECE-2, have so far been identified (Schmidt et al., 1994; Xu et al., 1994; Emoto & Yanagisawa, 1995). Enzyme activities of ECE-1 and ECE-2 are inhibited by phosphoramidon, a dual neutral endopeptidase (NEP)/ECE inhibitor, but not thiorphan, a NEP inhibitor (Turner & Murphy, 1996). Both ECEs are structurally related to NEP-24.11, and enzyme activity of NEP-24.11 is inhibited by phosphoramidon and thiorphan. NEP-24.11 is a plasma membrane-bound zinc metalloprotease that was initially isolated from renal epithelial brush border cells and cleaves peptide substances at the amino side of hydrophobic amino acids (Erdös & Skidgel, 1989). It is well known that NEP-24.11 efficiently cleaves potent vasoactive peptides such as ETs, substance P, bradykinin, arterial natriuretic peptide, C-type natriuretic peptide and angiotensins (Roques et al., 1993).
Recently, Hanson et al. (1997) reported that a serine protease in human lungs hydrolyzes big ET-1 to ET-11–31, which has a vasocontractile activity. Furthermore, it has been reported that human mast cell chymase specifically converts big ETs to ETs1–31 which have contractile activity in the trachea, without any further degradation products (Nakano et al., 1997). In the present study, we examined the effects of synthetic ET-11–31 and ET-21–31 on the intracellular free calcium concentration ([Ca2+]i) and [125I]-ET-1 binding using human cultured bronchial smooth muscle cells (BSMC). In addition, we analysed the mechanisms of the response to ET-11–31 and ET-21–31 using various protease inhibitors.
Methods
Cell culture
Human bronchial smooth muscle cells isolated from the bronchus of a 21-year-old male donor by the explant method were purchased from Clonetics (San Diego, U.S.A). The identity of human bronchial smooth muscle cells was verified by immunohistochemistry for α-smooth muscle actin. Greater than 95% of cells were α-actin smooth muscle immunopositive. The cells were cultured in MCDB131 medium supplemented with 5% foetal bovine serum, 0.5 ng ml−1 epidermal growth factor, 50 μg ml−1 insulin, 2 ng ml−1 fibroblast growth factor, 50 μg ml−1 gentamicin and 50 ng ml−1 amphotericin B. All experiments were performed with the cells in passages 6–7.
Measurement of [Ca2+]i
Cells were grown in 75-cm2 tissue culture flasks and removed from the flasks with 0.025% trypsin plus 0.01% EDTA. Cell suspensions were washed once with the culture medium. Cells were counted and resuspended in the culture medium to a final concentration of 106 cells ml−1. The cell suspensions were incubated with 1 μM fura-2/acetoxymethyl ester (fura-2/AM) at 37°C for 10 min. The loaded cells were resuspended in 0.5 ml of Tris-HEPES buffer solution (in mM:Tris 10, HEPES 10, KC1 5, MgCl2 1.2, CaCl2 1, NaCl 140, glucose 15, adjusted with HCl to pH 7.4) in a cuvette (50 ×7 mm diameter) and stirred continuously. Various concentrations of ET derivatives in 5 μl of 0.1% bovine serum albumin were added. ET receptor antagonist or protease inhibitor in 5 μl of 10% DMSO was added at 5 min before addition of ET derivatives. Fluorescence measurements were done with a spectrofluorometer (CAF-110, Japan Spectroscopy, Inc., Tokyo, Japan). [Ca2+]i was calculated according to the formula described by Grynkiewicz et al. (1985): [Ca2+]i=[(R−Rmin/Rmax−R)* (Sf/Sb)*KD, where R is the ratio of the 340/380 nm of the fluorescence signal, Rmin is the 340/380 ratio in calcium-free media with 10 mM EGTA added, Rmax is the 340/380 ratio in the presence of saturating calcium, and Sf/Sb is the ratio of the 380-nm fluorescence measured in a calcium-free solution to that measured in a calcium-replete solution. The KD value for fura-2/AM is 224 nM (Grynkiewicz et al., 1985).
Binding studies
[125I]-ET-1 and [125I]-ET-3 bindings to the cells were done as described previously (Mihara et al., 1994). The cells were cultured in 24-well culture plates. After 4–6 days, the culture medium was aspirated, and the cells were washed twice with ice-cold HEPES (20 mM)-buffered Hank's solution (pH 7.4). In the saturation experiments, the cells were incubated with increasing concentrations of [125I]-ET-1 and [125I]-ET-3 (10–700 pM) in 0.3 ml of HEPES-buffered Hank's solution containing 0.1 mM PMSF, 10 μg ml−1 aprotinin, 10 μg ml−1 leupeptin, 10 μg ml−1 pepstatin A, 250 μg ml−1 bacitracin and 10 μg ml−1 soybean trypsin inhibitor. Nonspecific bindings were determined with the same concentrations of iodinated ETs in the presence of 10−7 M unlabelled ETs. In the competition experiments, each well was incubated with 12.5 pM [125I]-ET-1 in the absence and presence of various concentrations of unlabelled ET-11–31, big ET-1, ET-21–31 or big ET-2. Nonspecific binding was defined in the presence of 10−7 M ET-1 and was about 5% of the total binding. Protease inhibitor was added at 20 min before addition of unlabelled ET derivatives. Equilibrium binding studies were performed at 37°C for 60 min. After rapid removal of the incubation medium, 0.25 ml of ice-cold HEPES-buffered Hank's solution was added. Free ligand was removed by washing the intact attached cells twice with ice-cold HEPES-buffered Hank's solution. Cells were dissolved in 0.1 N NaOH and transferred to a test tube, then the radioactivity was counted.
Drugs
Human ET-1, ET-11–31, big ET-1, ET-2, ET-21–31, big ET-2, ET-3 and phosphoramidon (N-(α-rhamnopyranosyloxyhydroxyphosphinyl)-L-leucyl-L-tryptophan) were obtained from Peptide Institute (Osaka, Japan). Fura-2/acetoxymethylester was purchased from Dojindo (Kumamoto, Japan). BQ123 and BQ788 were purchased from Neosystem Laboratoire (Strasbourg, France). [125I]-ET-1 and [125I]-ET-3 (81.4 Tbeq mmol−1) were obtained from New England Nuclear (Boston, MA, U.S.A.). Other reagents were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.).
Statistics
The results shown in the text, table and figures are expressed as mean values±s.e.mean. The potency of the agonists was assessed as the negative logarithm of the concentration required to cause 50% of the maximum response (pD2) using the logistic equation described by DeLean et al. (1978). Statistical analyses were made using Student's t-test and Tukey's method after one-way analysis of variance.
Results
Effect of ET derivatives on [Ca2+]i in human BSMC
ET-1 (3×10−10 M–10−7 M), ET-11–31 (10−8–3×10−7 M) and big ET-1 (3×10−8–10−6 M) induced a concentration-dependent increase in [Ca2+]i with pD2 values of 8.46±0.08 (n=3), 7.23±0.09 (n=3) and 6.76±0.02 (n=3), respectively (Figure 1A). Thus, the effect of ET-11–31 was about three times more potent than big ET-1, although it was about 17 times less potent than ET-1. As shown in Figure 1B, ET-2 (3×10−10–3×10−8 M) and ET-21–31 (3×10−8 M–3×10−6 M) also induced a concentration-dependent increase in [Ca2+]i, whereas big ET-2 (up to 10−6 M) did not show any increase in [Ca2+]i.
Figure 1.
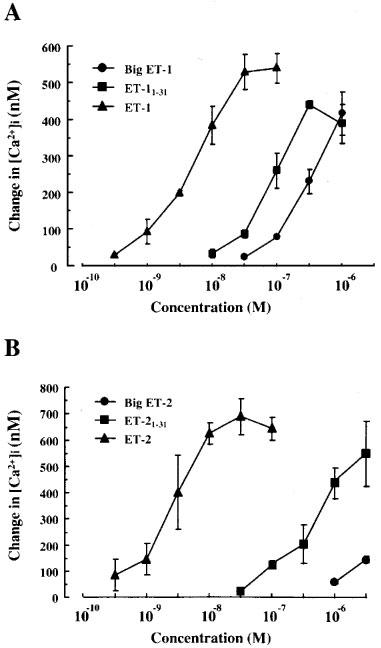
Concentration-response curves for increases in [Ca2+]i induced by ET-1, ET-11–31 and big ET-1 (A) and ET-2, ET-21–31 and big ET-2 (B) in human cultured BSMC. Changes in [Ca2+]i from basal level before addition of each stimulus were determined by fura-2/AM. Values are the mean±s.e.mean for three determinations.
ET receptor subtypes expressed on cultured human BSMC
To analyse ET receptor subtypes expressed on BSMC, we examined the effects of ETA- and ETB-receptor antagonists on the increase in [Ca2+]i induced by ET-1 or ET-3. As shown in Figure 2A, treatment with BQ123 (10−7 and 10−6 M) significantly inhibited the 10−7 M ET-1-induced increase in [Ca2+]i. Combined treatment with BQ123 (10−6 M) and BQ788 (10−6 M) suppressed the increase in [Ca2+]i induced by ET-1 more efficiently than treatment with BQ123 alone. On the other hand, 10−7 M ET-3 slightly induced a [Ca2+]i increase with about six times less potency than that of 10−7 M ET-1 (Figure 2B). BQ123 did not inhibit the increase in [Ca2+]i induced by ET-3, whereas BQ788 strongly suppressed that induced by ET-3.
Figure 2.

Effects of ETA- and ETB-receptor antagonists on [Ca2+]i increases induced by ET-1 (A) and ET-3 (B) in human cultured BSMC. Values are expressed as the change in [Ca2+]i from basal level before addition of each stimulus. The cell suspensions were treated for 5 min with BQ123 and BQ788 before addition of ET-1 (10−7 M) or ET-3 (10−7 M). Data are mean±s.e.mean (n=4). *P<0.05, **P<0.01 vs ET-1 or ET-3 by Tukey's method.
Effects of various protease inhibitors on [Ca2+]i increases induced by ETs1–31 and big ETs
As illustrated in Figure 3A, the increases in [Ca2+]i caused by 10−6 M ET-11–31 and 10−6 M big ET-1 were completely abolished by treatment with 10−4 M phosphoramidon. Similar inhibition by phosphoramidon was observed in the [Ca2+]i increase induced by the same concentration of ET-21–31 (Figure 3B, left). Although the effect of 10−6 M big ET-2 on [Ca2+]i was extremely weak, it was not affected by phosphoramidon (Figure 3B, right). These results are summarized in Figure 4. Increases in [Ca2+]i induced by ET-1 and ET-2 were not affected by 10−4 M phosphoramidon (data not shown).
Figure 3.
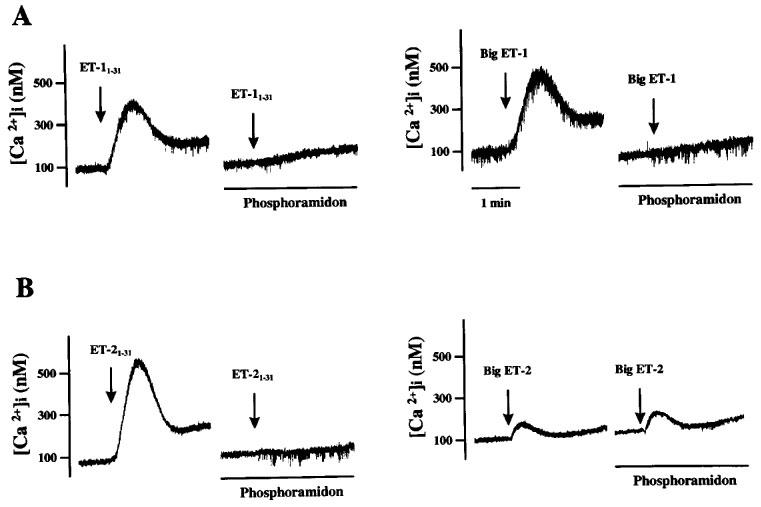
Representative charts for the effect of phosphoramidon on [Ca2+]i responses induced by ET-11–31, big ET-1 (A), ET-21–31 or big ET-2 (B) in human cultured BSMC. The cell suspensions were treated for 5 min with phosphoramidon (10−4 M) before addition of each stimulus. ET derivatives were added at 10−6 M.
Figure 4.

Effect of phosphoramidon on [Ca2+]i increases induced by ET-11–31, big ET-1 (A), ET-21–31 and big ET-2 (B) in human cultured BSMC. Values are expressed as the change in [Ca2+]i from the basal level before addition of each stimulus. Data are mean±s.e.mean (n=4). **P<0.01 vs control by Student's t-test.
To further characterize the phosphoramidon-sensitive converting enzyme that cleaves ETs1–31 and big ET-1 to corresponding ETs, the effects of various protease inhibitors on [Ca2+]i increases induced by big ET-1 and ET-21–31 were examined. As shown in Table 1, both increases were significantly inhibited by thiorphan (an inhibitor of NEP) but not BBI (a nonpermeable inhibitor of trypsin- and chymotrypsin-type proteases) or chymostatin (a permeable inhibitor of chymotrypsin-type proteases).
Table 1.
Effects of various protease inhibitors on [Ca2+]i increases induced by big ET-1 and ET-21–31 in human cultured BSMC

[125I]-ET-1 and [125I]-ET-3 binding
As shown in Figure 5, the binding of [125I]-ET-1 and [125I]-ET-3 to cultured BSMC was saturable. Scatchard plot analyses of these saturation curves showed that the apparent dissociation constant (KD) and maximal binding (Bmax) values were 63.8±6.9 pM and 29.6±0.7 fmol per 105 cells for [125I]-ET-1 binding, and 50.9±3.3 pM and 5.8±0.4 fmol per 105 cells for [125I]-ET-3 binding, respectively (Figure 5, inset). These data indicated that 80% of ET receptors were ETA-receptor subtype and 20% of those were ETB-receptor subtype in BSMC.
Figure 5.
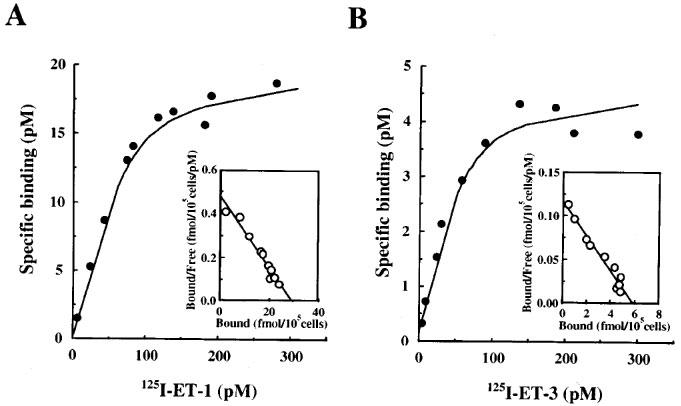
Saturation binding curves of [125I]-ET-1 (A) and [125I]-ET-3 (B) in human cultured BSMC. Scatchard plots for the specific bindings of [125I]-ET-1 and [125I]-ET-3 are shown in inset A and B, respectively. Binding analyses were performed on 105 cells. Each point represents the mean values of triplicate measurements.
In order to investigate the effect of thiorphan on the competition curves of ET-11–31, big ET-1, ET-21–31 and big ET-2, we examined the inhibition of the specific [125I]-ET-1 binding by various concentrations of these ET derivatives in the absence and presence of thiorphan (10−5 M). As shown in Figure 6A, the [125I]-ET-1 binding was concentration-dependently inhibited by ET-11–31 (3×10−9–10−7 M) or big ET-1 (10−8–10−6 M) in the absence of thiorphan. In the presence of thiorphan (10−5 M), these competition curves were shifted to the right. ET-21–31 (10−8–10−6 M) also inhibited the specific [125I]-ET-1 binding concentration-dependently in the absence of thiorphan, whereas the inhibitory effect of big ET-2 on the binding was extremely weak compared to that of ET-21–31. Thiorphan (10−5 M) strongly suppressed the effect of ET-21–31, but did not that of big ET-2 (Figure 6B). Thiorphan (10−5 M) did not affect the competition curves of ET-1 and ET-2 (data not shown). Phosphoramidon (10−4 M) also inhibited the effects of ET-11–31, big ET-11–31 and ET-21–31, but not of big ET-2 (data not shown).
Figure 6.
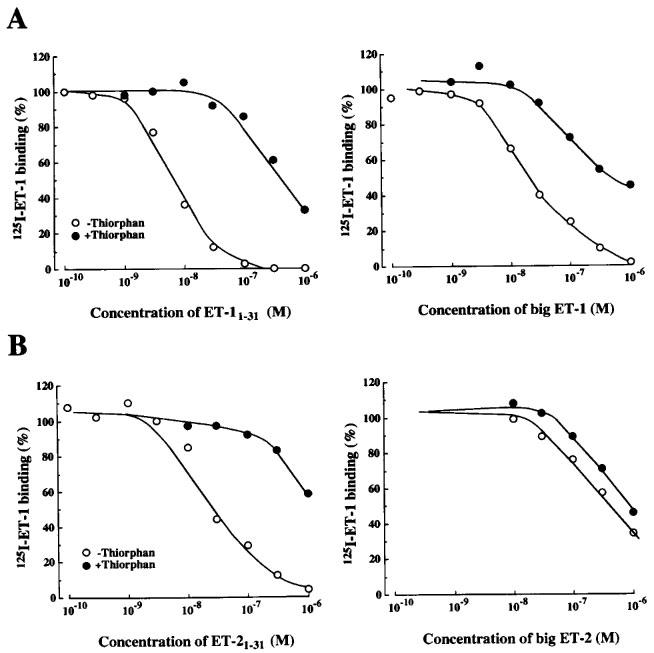
Effects of the absence or presence of thiorphan (10−5 M) on the inhibition of [125I]-ET-1 binding by ET-11–31, big ET-1 (A), ET-21–31, and big ET-2 (B) in human cultured BSMC. Each point represents the mean values of triplicate determinations and are representative of those from three separate experiments.
Discussion
The physiological actions of ETs are mediated by at least two distinct receptor subtypes, ETA- and ETB-receptors. ETA-receptor has a higher affinity for ET-1 and ET-2 than for ET-3, whereas ETB-receptor has an equal affinity for all isopeptides (Arai et al., 1990; Sakurai et al., 1990). In human cultured BSMC, an increase in [Ca2+]i caused by a high concentration of ET-1 was inhibited by treatment with BQ123, an ETA-receptor antagonist, in a concentration-dependent manner. Combined treatment with BQ123 and BQ788, an ETB-receptor antagonist, suppressed the increase in [Ca2+]i caused by ET-1 more efficiently than treatment with BQ123 alone. Although a high concentration of ET-3 also increased [Ca2+]i, the effect was about six times less than that of the same concentration of ET-1. The increase in [Ca2+]i caused by ET-3 was not affected by treatment with BQ123 but strongly suppressed by BQ788. Furthermore, Scatchard analyses of the saturation curves of [125I]-ET-1 and [125I]-ET-3 bindings to the cells showed that BSMC expressed both ETA- and ETB-receptors, and the calculated ratio of the ETA- to ETB-receptor number was about 4 : 1. These results suggest that the increases in [Ca2+]i induced by ET-1 and ET-2 are mainly mediated by ETA-receptor on human BSMC.
ET-11–31 and big ET-1 caused an increase in [Ca2+]i in a concentration-dependent manner, and the effect of ET-11–31 was about three times more potent than big ET-1 in human BSMC. On the other hand, ET-21–31 also caused an increase in [Ca2+]i, but big ET-2 at concentrations up to 10−6 M did not. Lebel et al. (1996) have reported that the contractions of strips in guinea-pig lung parenchyma elicited by big ET-1 and big ET-2 result from their conversion to ET-1 and ET-2 by NEP. Laporte & Sirois (1997) have also reported that the generation of ET-1 and ET-2 from exogenous big ET-1 and big ET-2 are suppressed by phosphoramidon, a dual NEP/ECE inhibitor, and thiorphan, a NEP inhibitor, in cultured guinea-pig Clara cells. Thus, we examined the effects of these protease inhibitors on the increases in [Ca2+]i caused by ET derivatives. Treatment with phosphoramidon almost completely inhibited the increases in [Ca2+]i caused by ET-11–31, big ET-1 and ET-21–31. Furthermore, the increases in [Ca2+]i by big ET-1 and ET-21–31 were strongly suppressed by thiorphan, but not by BBI or chymostatin. In addition to these functional studies, radio-ligand binding studies showed that the inhibitory effects of ET-11–31, big ET-1 and ET-21–31 on the [125I]-ET-1 binding were strongly suppressed by treatment with thiorphan and phosphoramidon. Taken together with previous reports, our results strongly suggest that the increases in [Ca2+]i caused by ET-11–31, big ET-1 and ET-21–31 are mediated through cleavage to these corresponding ETs by NEP in human BSMC. Our results are also supported by the reports that ECE requires the carboxyl-terminal sequence from His27 to Gly34 and Trp21 of big ET-1 for enzyme recognition and is not able to cleave ET-11–31 (Ohnaka et al., 1993; Xu et al., 1994).
In the present study, big ET-2 of up to 10−6 M did not cause any increase in [Ca2+]i, but ET-21–31 did in BSMC. Furthermore, the inhibitory effect of big ET-2 to [125I]-ET-1 binding was not affected by treatment with thiorphan. Thus, it appears that ET-21–31 is efficiently converted to ET-2 by NEP, but big ET-2 is not. Erdös & Skidgel (1989) reported that NEP cleaves peptide substances at the amino side of hydrophobic amino acids. Further studies are needed to clarify the substrate specificity for NEP.
In human BSMC, ET-1 and ET-2 in concentrations ranging from 3×10−10–10−7 M caused an increase in [Ca2+]i in a concentration-dependent manner. These concentrations, needed to induce functional action in this study, agreed well with those of a previous report that ET-1 induces the elevation of [Ca2+]i and causes an increase in the c-fos and c-myc mRNA levels in rat vascular smooth muscle cells (Komuro et al., 1988). In human coronary artery smooth muscle cells, however, Yoshizumi et al. (1998) reported that ET-1 (10−14–10−10 M) causes an increase in [Ca2+]i with a concentration-dependent manner. They showed that ET-11–31 (10−13–10−10 M) by itself causes an increase in [Ca2+]i mediated through ETA-receptor or an ETA-like receptor since the effect of ET-11–31 is not affected by phosphoramidon. The discrepancy in the sensitivity of ET-1 between their report and ours may arise from the differences in the [Ca2+]i measuring method or among smooth muscle cell types. However, at least we could not observe any direct effect of ETs1–31 in human BSMC.
Human chymase has been reported to be highly efficient for converting angiotensin I to angiotensin II (Urata et al., 1990b), and the chymase-dependent angiotensin II-forming pathway is recognized as a major pathway for angiotensin II formation in cardiovascular tissues (Urata et al., 1990a). Recently, Nakano et al. (1997) reported that human mast cell chymase also specifically converts big ETs to ETs1–31, without any further degradation products. In asthmatic patients with bronchial hyper-responsiveness, ET-like immunoreactivity strikingly increases in the airway epithelium and bronchoalveolar lavage fluid, and activated mast cells also increase in number in the bronchial tissue and bronchial lavage fluid (Nomura et al., 1989; Mattoli et al., 1991; Springall et al., 1991). Chymase-like immunoreactivity and enzymatic activity have been demonstrated in not only cardiovascular tissues but also human lung tissue (Urata et al., 1993). These reports lead us to the hypothesis that human chymase released from mast cells by various stimuli converts big ETs to ETs1–31. Recently, Takai et al. (1998) reported that the contractile response of big ET-2 is predominantly dependent on the conversion of big ET-2 to ET-21–31 by chymase in isolated monkey trachea. Human chymase seems to play an important role in the ET (especially ET-2) generating pathway in inflammatory tissues. In human BSMC, we showed that the increases in [Ca2+]i caused by ET-11–31, big ET-1 and ET-21–31 are mediated through corresponding ETs cleaved by NEP. NEP has been found in various types of cells, such as human airway smooth muscle and rat aortic fibroblasts and smooth muscle cells (Hamad et al., 1997; González et al., 1998). Therefore, it is likely that local production of ETs through ETs1–31 is regulated by the NEP cleavage pathway.
In this report, we presented the first evidence that ET-11–31 and ET-21–31 cause an increase in [Ca2+]i by being converted into the corresponding ET-1 and ET-2 by NEP in human BSMC. Human chymase may play an important role in the ET generating pathway in bronchial tissue.
Abbreviations
- BSMC
bronchial smooth muscle cells
- [Ca2+]i
intracellular free Ca2+ concentration
- ECE
endothelin-converting enzyme
- ET
endothelin
- NEP
neutral endopeptidase
References
- ARAI H., HORI S., ARAMORI I., OHKUBO H., NAKANISHI S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- DELEAN A., MUNSON P.J., RODBARD D. Simultaneous analysis of families of sigmoidal curves: applications to bioassay, radioligand assay and physiological dose response curves. Am. J. Physiol. 1978;235:E97–E102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- EMOTO N., YANAGISAWA M. Endothelin-converting enzyme-2 is a membrane-bound, phosphoramidon-sensitive metalloprotease with acidic pH optimum. J. Biol. Chem. 1995;270:15262–15268. doi: 10.1074/jbc.270.25.15262. [DOI] [PubMed] [Google Scholar]
- ERDÖS E.G., SKIDGEL R.A. Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB J. 1989;3:145–151. [PubMed] [Google Scholar]
- GONZÁLEZ W., SOLEILHAC J.M., FOURNIÉ-ZALUSKI M.C., ROQUES B.P., MICHEL J.B. Characterization of neutral endopeptidase in vascular cells, modulation of vasoactive peptide levels. Eur. J. Pharmacol. 1998;345:323–331. doi: 10.1016/s0014-2999(98)00038-7. [DOI] [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- HAMAD A.M., RANGE S., HOLLAND E., KNOX A.J. Regulation of cGMP by soluble and particulate guanylyl cyclases in cultured human airway smooth muscle. Am. J. Physiol. 1997;273:L807–L813. doi: 10.1152/ajplung.1997.273.4.L807. [DOI] [PubMed] [Google Scholar]
- HANSON G.C., ANDERSSON K.E., GYLLSTEDT E., HÖGESTÄTT E.D., LINDBERG B.F. Hydrolysis of big endothelin-1 by a serine protease in the membrane fraction of human lung. Regul. Pept. 1997;68:63–69. doi: 10.1016/s0167-0115(96)02105-2. [DOI] [PubMed] [Google Scholar]
- INOUE A., YANAGISAWA M., KIMURA S., KASUYA Y., MIYAUCHI T., GOTO K., MASAKI T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc. Natl. Acad. Sci. U.S.A. 1989;86:2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMURO I., KURIHARA H., SUGIYAMA T., TAKAKU F., YAZAKI Y. Endothelin stimulates c-fos and c-myc expression and proliferation of vascular smooth muscle cells. FEBS lett. 1988;238:249–252. doi: 10.1016/0014-5793(88)80489-7. [DOI] [PubMed] [Google Scholar]
- LAPORTE J., SIROIS P. Phosphoramidon and thiorphan suppress the generation of endothelin (ET) from exogenous big-endothelin by guinea pig Clara cells. Regul. Pept. 1997;68:105–109. doi: 10.1016/s0167-0115(96)02111-8. [DOI] [PubMed] [Google Scholar]
- LEBEL N., D'ORLÉANS-JUSTE P., FOURNIER A., SIROIS P. Role of the neutral endopeptidase 24.11 in the conversion of big endothelins in guinea-pig lung parenchyma. Br. J. Pharmacol. 1996;117:184–188. doi: 10.1111/j.1476-5381.1996.tb15172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTOLI S., SOLOPERTO M., MARINI M., FASOLI A. Levels of endothelin in the bronchoalveolar lavage fluid of patients with symptomatic asthma and reversible airflow obstruction. J. Allergy Clin. Immunol. 1991;88:376–384. doi: 10.1016/0091-6749(91)90100-3. [DOI] [PubMed] [Google Scholar]
- MIHARA S., NAKAJIMA S., MATSUMURA S., KOHNOIKE T., FUJIMOTO M. Pharmacological characterization of a potent nonpeptide endothelin receptor antagonist, 97-139. J. Pharmacol. Exp. Ther. 1994;268:1122–1128. [PubMed] [Google Scholar]
- NAKANO A., KISHI F., MINAMI K., WAKABAYASHI H., NAKAYA Y., KIDO H. Selective conversion of big endothelins to tracheal smooth muscle-constricting 31-amino acid-length endothelins by chymase from human mast cells. J. Immunol. 1997;159:1987–1992. [PubMed] [Google Scholar]
- NOMURA A., UCHIDA Y., KAMEYAMA M., SAOTOME M., OKI K., HASEGAWA S. Endothelin and bronchial asthma. Lancet. 1989;2:747–748. doi: 10.1016/s0140-6736(89)90814-3. [DOI] [PubMed] [Google Scholar]
- OHNAKA K., TAKAYANAGI R., NISHIKAWA M., HAJI M., NAWATA H. Purification and characterization of a phosphoramidon-sensitive endothelin-converting enzyme in porcine aortic endothelium. J. Biol. Chem. 1993;268:26759–26766. [PubMed] [Google Scholar]
- ROQUES B.P., NOBLE F., DAUGÉ V., FOURNIÉ-ZALUSKI M.C., BEAUMONT A. Neutral endopeptidase 24.11: structure, inhibition, and experimental and clinical pharmacology. Pharmacol. Rev. 1993;45:87–146. [PubMed] [Google Scholar]
- SAKURAI T., YANAGISAWA M., TAKUWA Y., MIYAZAKI H., KIMURA S., GOTO K., MASAKI T. Cloning of cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- SCHMIDT M., KRÖGER B., JACOB E., SEULBERGER H., SUBKOWSKI T., OTTER R., MEYER T., SCHMALZING G., HILLEN H. Molecular characterization of human and bovine endothelin converting enzyme (ECE-1) FEBS lett. 1994;356:238–243. doi: 10.1016/0014-5793(94)01277-6. [DOI] [PubMed] [Google Scholar]
- SPRINGALL D.R., HOWARTH P.H., COUNIHAN H., DJUKANOVIC R., HOLGATE S.T., POLAK J.M. Endothelin immuno- reactivity of airway epithelium in asthmatic patients. Lancet. 1991;337:697–701. doi: 10.1016/0140-6736(91)90279-x. [DOI] [PubMed] [Google Scholar]
- TAKAI S., SHIOTA N., JIN D., MIYAZAKI M. Chymase processes big-endothelin-2 to endothelin-2-(1-31) that induces contractile responses in the isolated monkey trachea. Eur. J. Pharmacol. 1998;358:229–233. doi: 10.1016/s0014-2999(98)00622-0. [DOI] [PubMed] [Google Scholar]
- TURNER A., MURPHY L.J. Molecular pharmacology of endothelin converting enzymes. Biochem. Pharmacol. 1996;51:91–102. doi: 10.1016/0006-2952(95)02036-5. [DOI] [PubMed] [Google Scholar]
- URATA H., BOEHM K.D., PHILIP A., KINOSHITA A., GABROVSEK J., BUMPUS F.M., HUSAIN A. Cellular localization and regional distribution of an angiotensin II-forming chymase in the heart. J. Clin. Invest. 1993;91:1269–1281. doi: 10.1172/JCI116325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- URATA H., HEALY B., STEWART R.W., BUMPUS F.M., HUSAIN A. Angiotensin II-forming pathways in normal and failing human hearts. Circ. Rec. 1990a;66:883–890. doi: 10.1161/01.res.66.4.883. [DOI] [PubMed] [Google Scholar]
- URATA H., KINOSHITA A., MISONO K.S., BUMPUS F.M., HUSAIN A. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J. Biol. Chem. 1990b;265:22348–22357. [PubMed] [Google Scholar]
- XU D., EMOTO N., GIAID A., SLAUGHTER C., KAW S., DEWIT D., YANAGISAWA M. ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell. 1994;78:473–485. doi: 10.1016/0092-8674(94)90425-1. [DOI] [PubMed] [Google Scholar]
- YOSHIZUMI M., INUI D., OKISHIMA N., HOUCHI H., TSUCHIYA K., WAKABAYASHI H., KIDO H., TAMAKI T. Endothelin-1-(1-31), a novel vasoactive peptide, increases [Ca2+]i in human coronary artery smooth muscle cells. Eur. J. Pharmacol. 1998;348:305–309. doi: 10.1016/s0014-2999(98)00158-7. [DOI] [PubMed] [Google Scholar]


