Abstract
This study aimed to characterize for the first time in vitro 5-HT4 receptors in the canine gastrointestinal tract. For this purpose, we used circular muscle strips of the canine isolated rectum.
In the presence of methysergide (60 μM), 5-HT induced relaxation of methacholine (1 μM)-precontracted muscle strips, yielding a monophasic sigmoidal concentration-relaxation curve (pEC50 7.2±0.07).
Tetrodotoxin (0.3 μM) did not affect the curve to 5-HT, suggesting the inhibitory 5-HT receptor is located on the smooth muscle. Granisetron (0.3 μM) did also not affect the curve to 5-HT, which excludes the 5-HT3 receptor mediating the relaxation to 5-HT. The presence of methysergide rules out the involvement of 5-HT1, 5-HT2 or 5-HT7 receptors.
5-HT, the selective 5-HT4 receptor agonists R076186, prucalopride (R093877) and SDZ HTF-919 and the 5-HT4 receptor agonists cisapride and 5-MeOT relaxed the muscle strips with a rank order of potency R076186=5-HT>cisapride>prucalopride⩾SDZ HTF-919>5-MeOT.
The selective 5-HT4 receptor antagonists GR 125487, RS 39604 and GR 113808 competitively antagonized the relaxations to 5-HT, yielding pKB estimates of 9.7, 7.9 and 9.1, respectively. The selective 5-HT4 receptor antagonist SB 204070 shifted the curve to 5-HT rightward and depressed the maximal response (apparent pA2 10.6). GR 113808 (10 nM) produced a parallel rightward shift of the curve to the selective 5-HT4 receptor agonists R076186 (pA2 8.8).
It is concluded that 5-HT induces relaxation of the canine rectum circular muscle through stimulation of a single population of smooth muscle 5-HT4 receptors. For the first time, a non-human species was shown to exhibit relaxant 5-HT4 receptors in the large intestine.
Keywords: 5-HT4 receptors, relaxation, 5-hydroxytryptamine, canine, rectum, colon, large intestine
Introduction
5-HT4 receptors are abundantly distributed along the gastrointestinal tract, where they may play a role in modulating smooth muscle tone, peristaltic reflex and mucosal secretion (see Hegde & Eglen, 1996; Grider et al., 1998). In the clinic, 5-HT4 receptor agonists (for example cisapride) are used to relieve patients suffering from gastro-oesophageal reflux diseases, dyspepsia or gastroparesis (see Briejer et al., 1995) and putatively, they could be indicated for constipation (for example prucalopride; R093877; Briejer et al., 1998b). 5-HT4 receptor antagonists might be useful in the treatment of irritable bowel syndrome (Sanger, 1996).
In studies measuring motility in the dog in vivo, colonic (Nagakura et al., 1996; Briejer et al., 1998a) and gastric (Bingham et al., 1995) 5-HT4 receptor-mediated effects have been identified. However, using in vitro methods, canine 5-HT4 receptors have not been characterized yet.
In our previous attempts to characterize the effects of 5-HT in the canine large intestine, we found contractile 5-HT2A receptors on colonic longitudinal muscle (Prins et al., 1997). Furthermore, in colonic circular muscle we identified 5-HT2-like receptors involved in the amplification of carbachol-induced contractions (Prins et al., 1998a). The latter phenomenon was blocked by methysergide (60 μM), revealing a 5-HT-induced relaxation. This 5-HT-induced relaxation of the circular muscle along the large intestine was most pronounced in the distal part, the rectum. This study aims to characterize pharmacologically the 5-HT receptor mediating the relaxation to 5-HT in the canine rectum.
Methods
Beagle dogs of both sexes, weighing 7–14 kg, were used. They were previously used in studies to assess the cardiovascular effects of compounds in vivo. They were anaesthetized with pentobarbital (30 mg kg−1 i.v.), and, subsequently, sacrificed with KCl (150 mg kg−1 i.v.). The abdomen was incised and a segment of rectum of approximately 4 cm, at the point where the rectum is bound to the urethra (male) and the vagina (female) was dissected. For one set of experiments, to compare regional differences in response to 5-HT, ascending colon and descending colon and rectum were used. The segment was cut open longitudinally, luminal contents were rinsed out with modified Krebs-Henseleit solution containing (mM): glucose 5.55, CaCl2 2.51, NaHCO3 25, MgSO4 1.18, KH2PO4 1.18, KCl 4.69 and NaCl 118, and the mucosa and mesentery were removed. The longitudinal muscle layer was carefully dissected and four strips of circular muscle were cut, each with a length of half the circumference (approx. 2–3 cm). The strips were anchored to organ bath hooks and mounted in a classical organ bath set-up for isotonic measurement (2 g load), filled with modified Krebs-Henseleit solution of 37°C and gassed with carbogen (95% O2, and 5% CO2).
Experimental protocol
After two washouts and 15 min of stabilization time, the strips were contracted four times with methacholine (MCh 1 μM, approximately EC50). Each dose of MCh (1 μM) was left in the organ bath for 15 min, followed by three wash-outs, of which two were applied immediately. The third was applied 10 min later and was followed by a 5 min period of stabilization, before the next dose of MCh (1 μM) was given.
To block 5-HT-induced contractions, methysergide (60 μM) was routinely added. Antagonist or solvent were administered hereafter and left to equilibrate for 30 min. Then, the strips were contracted with MCh (1 μM; taken as 100%) and, after a stable contraction had been established (after approximately 25 min), 5-HT or 5-HT4 receptor agonists were added to the organ bath solution in a log unit incrementing cumulative interval. One curve was made per strip and only one control (=solvent) curve to 5-HT was established per dog.
Preliminary experiments revealed that methysergide (60 μM) induced an inhibition of spontaneous contractility, allowing the assessment of full range concentration-response curves. Furthermore, it was found that methysergide did not significantly affect the concentration-relaxation curve to the selective 5-HT4 receptor agonist R076186 (n=4; results not shown). Methysergide, therefore, was considered a useful tool to isolate and investigate 5-HT4 receptor-mediated responses. Under the applied conditions, 5-HT (10 μM) induced relaxations of ascending colon (6±2%), descending colon (45±2%) or rectum (59±5%). These 5-HT-induced relaxations were antagonized by the selective 5-HT4 receptor antagonist GR 113808 (30 nM), resulting in a blockade of the relaxation in the ascending colon, and an inhibition of the relaxation in the descending colon and the rectum (n=2–6).
Data analysis
For analysis and graphical presentation, the MCh (1 μM)-induced contraction, which was established prior to the dosing cycle of agonist, was taken as 100% contraction, and relaxations to agonists were expressed as percentage of that contraction ± standard error of the mean (s.e.mean.). The data points were iteratively fitted to the Hill equation, obtaining estimates for the mid-point location (pEC50), the Hill slope (nH) and the maximum effect for that specific agonist (α). The intrinsic activity of agonists was estimated by relating the maximum response to the agonist under study to the maximum response to 5-HT, which in this bioassay proved to be the agonist producing relatively the greatest relaxation.
Antagonist affinities were estimated by fitting the pEC50 estimates simultaneously to the Schild equation (according to the method of Black et al., 1985), obtaining the estimate for pKB. If the criteria for competitive antagonism were not met (i.e. antagonist-induced change in nH or α), the apparent antagonist affinity was estimated using the Schild equation for the lowest concentration of antagonist that significantly shifted the curve to the agonist rightward, providing the apparent pA2. If only one concentration of antagonist was tested, which produced a parallel, dextral rightward shift of the curve to 5-HT, the Schild equation was used to estimate a pA2 (Arunlakshana & Schild, 1959).
Statistical analysis
To test the criteria for Schild-analysis, ANOVA was performed, followed by a post-hoc Bonferroni's test for multiple comparisons. For single comparisons, a Student's t-test was performed, as appropriate. A level of P<0.05 was considered to indicate statistically significant difference. The number of dogs used for each experiment is denoted by n.
Compounds
The following compounds were used (with their abbreviations, if any, in italics, and respective suppliers between parentheses): 5-methoxytryptamine (5-MeOT), (1-butyl-4-piperidinyl)-methyl - 8 - amino - 7 - chloro - 1,4 - benzodioxane-5-carboxylate HCl (SB 204070), 1-[2-[(methylsulphonyl)amino] ethyl]-4-piperidinyl-methyl 5-fluoro-2-methoxy-1H-indole-3-carboxylate (GR 125487), [1-[2-[(methylsulphonyl)amino]ethyl]-4- piperidinyl]methyl 1-methyl-1H-indole-3-carboxylate (GR 113808), granisetron HCl, cisapride monohydrate, 5-methoxy-indole-3-carboxaldehyde amino(pentylamino) methylene hydrazone hydrogenmaleate (SDZ HTF-919), 4-amino-5-chloro - 2,3 - dihydro-N-(1-[3-methoxypropyl]-4-piperidinyl)-7-benzofurancarboxamide HCl (prucalopride; R093877), cis-4-amino - 5 - chloro -N-[1-[4-[4-(dimethylamino)-1-piperidinyl]-4-oxo - butyl] - 3- methoxy - 4 - piperidinyl]- 2-methoxybenzamide (R076186; Janssen Research Foundation, Belgium), tetrodotoxin, 5-HT creatinine sulphate, (Serva, Germany), methysergide maleate (Sandoz, Switzerland), pargyline HCl (Abbott, U.S.A.), methacholine HCl (MCh), cocaine HCl (Merck, Germany), reboxetine methanesulphonate (Farmitalia Carlo Erba, Italy), 1-(4-amino-5-chloro-2-(3,5-dimethoxy)benzyloxyphenyl)-3-[1-((2-methylsulphonylamino)ethyl)piperidin-4 -yl]-1-propanone (RS 39604; Merck Belgolabo, Belgium), pentobarbital (Nembutal® 60 mg ml−1, Sanofi, Belgium).
All compounds were dissolved in 0.9% NaCl solution, except for cisapride, GR 113808, R076186 and reboxetine, which were dissolved in 0.9% NaCl acidified with tartaric acid in the stock solution, pargyline, that was dissolved in distilled water with 10% cyclodextrine in the stock solution and SDZ HTF-919, that was dissolved in distilled water with 10% cyclodextrine acidified with tartaric acid in the stock solution. The solvents had no effect on the muscle strips per se. All stock solutions were prepared freshly on the day of the experiment and dilutions were prepared using 0.9% NaCl solution.
Results
Initially, the strips displayed spontaneous contractility. MCh (1 μM; EC50) induced a contraction that stabilized after approximately 25 min. The spontaneous contractility (10–20% of the MCh (1 μM)-induced contraction) progressively reduced after four consecutive administrations of MCh (1 μM). The consecutive contractions to MCh (1 μM) were stable. 5-HT concentration-dependently induced relaxations of the rectal muscle strips yielding a monophasic sigmoidal concentration-relaxation curve (pEC50 7.2±0.08, n=9; Figure 1). The Na+ channel blocker tetrodotoxin (0.3 μM; n=6; results not shown) did not affect the concentration-relaxation curve to 5-HT, suggesting the receptor is located on smooth muscle cells. Inhibition of re-uptake-1 by cocaine (30 μM), of selective 5-HT re-uptake by fluoxetine (0.3 μM), of monoamine oxidase by pargyline (0.1 mM) or of noradrenaline re-uptake by reboxetine (1 μM) did not alter the concentration-response curve to 5-HT (n=6; results not shown). Tetrodotoxin or inhibitors of uptake or breakdown were, therefore, not included in the organ bath solution routinely.
Figure 1.
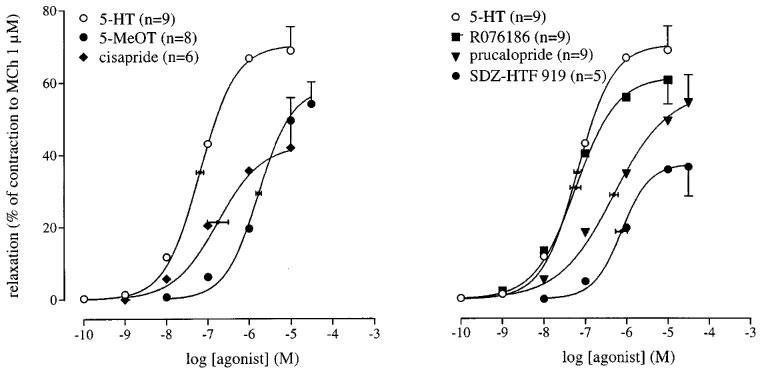
Concentration-relaxation curves to 5-HT, 5-MeOT and cisapride (left panel) and to 5-HT, R076186, prucalopride and SDZ HTF-919 (right panel) in the canine isolated rectal circular smooth muscle. The curves shown superimposed on the mean experimental data points represent simulations using the Hill-equation and the parameters for midpoint location (with horizontal standard error bars), upper asymptote location (with vertical standard error bars) and the Hill slope, that were obtained from the iterative fitting procedure.
5-HT, the selective 5-HT4 receptor agonists R076186, SDZ HTF-919 and prucalopride, and the 5-HT4 receptor agonists cisapride and 5-MeOT all induced relaxation of the muscle strips (Figure 1). 5-HT was the most efficacious agonist and the maximum relaxations to the selective 5-HT4 receptor agonists R076186 and prucalopride, the 5-HT4 receptor agonists cisapride and 5-MeOT were not significantly different from that obtained by 5-HT (reflected in the intrinsic activity; see Table 1). The selective 5-HT4 receptor agonist SDZ HTF-919 yielded an intrinsic activity that was significantly less than that obtained by 5-HT. Prucalopride and SDZ HTF-919 behaved as approximately equipotent agonists. The rank order of agonist potency (with the concomitant pEC50 values between parentheses) was R076186 (7.2)=5-HT (7.2)>cisapride (6.8)>prucalopride (6.3) ⩾ SDZ HTF-919 (6.1) > 5-MeOT (5.8). Prucalopride, SDZ HTF-919, R076186, 5-MeOT and cisapride, but not 5-HT produced a second, low-affinity phase at concentrations exceeding 30 μM. At concentrations of approximately 300 μM of the above-mentioned agonists, the muscle strips relaxed up to 100% of the precontraction. This low-affinity phase appeared to be non-5-HT4 receptor-mediated, as GR 113808 (in excess of 1 μM) shifted the high-affinity phase of the curve to R076186 rightward, leaving the low-affinity phase unaffected (n=2–4; results not shown). Investigation on the nature of the low-affinity phase fell outside the scope of this paper and was not further pursued.
Table 1.
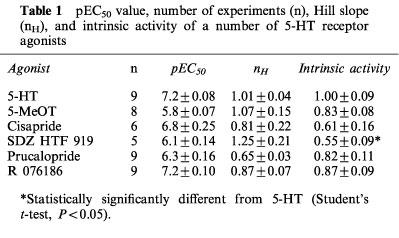
pEC50 value, number of experiments (n), Hill slope (nH), and intrinsic activity of a number of 5-HT receptor agonists
The selective 5-HT3 receptor antagonist granisetron (0.3 μM) did not alter the concentration-relaxation curve to 5-HT (pEC50 7.3±0.1; n=6; results not shown), which suggests that 5-HT3 receptors are not involved in the 5-HT-induced relaxation. In contrast, the selective 5-HT4 receptor antagonists GR 113808 (10, 30 and 100 nM), RS 39604 (0.1, 0.3, 1 and 3 μM) and GR 125487 (0.3, 1 and 3 nM) all produced a parallel rightward displacement of the concentration-relaxation curve to 5-HT, yielding Schild plots with slopes that were not significantly different from unity (Figure 2; Table 2). After constraining the Schild slopes to unity, pKB estimates of 9.1±0.11 (GR 113808), 9.7±0.07 (GR 125487) and 7.9±0.05 (RS 39604) were obtained. The selective 5-HT4 receptor antagonist SB 204070 (0.1, 0.3 and 1 nM) failed to meet the criteria for competitive antagonism (Figure 3), displaying a concentration-dependent rightward shift and a concomitant depression of the curve to 5-HT. Therefore, an apparent pA2 value of 10.6±0.17 was estimated, using only the rightward shift induced by SB 204070 (0.1 nM). The Schild slopes and affinity estimates obtained are given in Table 2.
Figure 2.
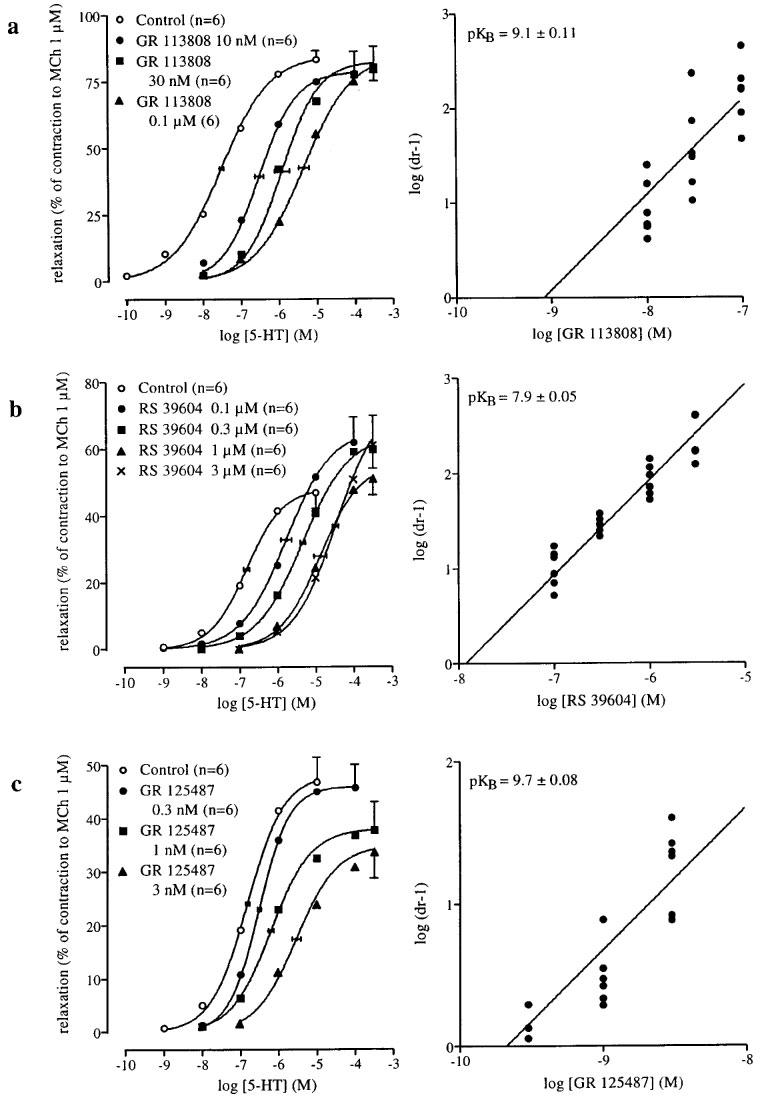
Left panels: Concentration-relaxation curves to 5-HT in the absence and presence of GR 113808 (a), RS 39604 (b), and GR 125487 (c) in the canine isolated rectal circular smooth muscle. The curves shown superimposed on the mean experimental data points are simulations using the Hill-equation and the parameters for midpoint location (with horizontal standard error bars), upper asymptote location (with vertical standard error bars) and the Hill slope that were obtained from the iterative fitting procedure. Right panels: The Schild plots for GR 113808 (a), RS 39604 (b) and GR 125487 (c). The line shown superimposed on the experimental data points was obtained by stimulating Schild regression analysis with the Schild slope constrained to unity, providing the X-axis intercept representing the pKB.
Table 2.
Schild slopes with 95% confidence limits (CL), affinity estimates (pA2/pKB values) and literature affinities of selective 5-HT4 receptor antagonists
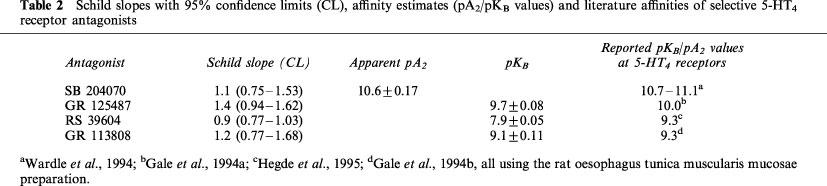
Figure 3.
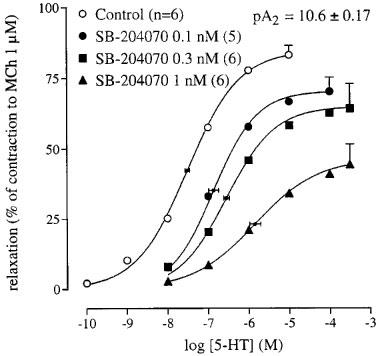
The concentration-relaxation curves to 5-HT in the absence and presence of SB 204070 in the canine isolated rectal circular smooth muscle. The curves shown superimposed on the mean experimental data points are simulations using the Hill-equation and the parameters for midpoint location (with horizontal standard error bars), upper asymptote location (with vertical standard error bars) and the Hill slope that were obtained from the iterative fitting procedure.
GR 113808 (10 nM) produced a significant rightward shift of the curve to R076186. Using GR 113808 (30 nM), concentrations of R076186 higher than 30 μM had to be administered to reach the maximum relaxation. This resulted in a deformation of the curve to R076186 due to the second phase, therefore, it was not feasible to estimate a pKB for GR 113808 against R076186. Using only the rightward shift of the curve to R076186 produced by GR 113808 (10 nM), an pA2 of 8.8±0.11 was estimated (Figure 4).
Figure 4.
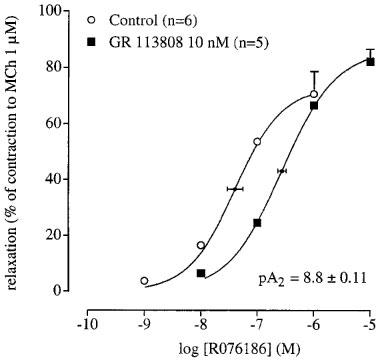
The concentration-relaxation curves to R076186 in the absence and presence of GR 113808 using the canine isolated rectal circular smooth muscle preparation. The curves shown superimposed on the mean experimental data points are simulations using the Hill-equation and the parameters for midpoint location (with horizontal standard error bars), upper asymptote location (with vertical standard error bars) and the Hill slope that were obtained from the iterative fitting procedure.
Discussion
The data of the present study clearly suggest that under the applied conditions, functional 5-HT4 receptors mediate relaxation of the canine rectum circular smooth muscle in vitro. This is the first report to describe the characterization of functional canine 5-HT4 receptors in vitro.
The rank order of potency of the 5-HT agonists was consistent with a 5-HT4 receptor (Figure 1; Table 1). R076186 (Briejer et al., 1993), prucalopride (Briejer et al., 1998b) and SDZ HTF-919 (Buchheit et al., 1995) are potent and selective 5-HT4 receptor agonists, and they all produced relaxations at 5-HT4 receptor-selective concentrations. Cisapride has affinity for a number of 5-HT receptors, such as 5-HT2A and 5-HT3 receptors (Gommeren et al., 1998) but is an agonist only at 5-HT4 receptors (Briejer et al., 1993). Indeed, in the assay under study, cisapride was a relatively potent agonist. 5-MeOT was found to be less potent than 5-HT. This is in accordance with reported data showing that 5-MeOT is less potent than 5-HT (Gale et al., 1994b), using the rat oesophagus muscularis mucosae preparation, although another report shows that 5-MeOT and 5-HT were equipotent at rat oesophageal 5-HT4 receptors (Hegde & Eglen, 1996). Taken together, the agonist rank order of potency strongly suggests that the relaxation is due to activation of 5-HT4 receptors.
The affinity estimates of the selective and highly potent 5-HT4 receptor antagonists GR 113808 and GR 125487 (9.1 and 9.7, respectively; Figure 2 and Table 2) were in good accordance with affinities reported previously (GR 113808: Gale et al., 1994b; GR 125487: Gale et al., 1994a). The selective and highly potent 5-HT4 receptor antagonist SB 204070 (Wardle et al., 1994) was a ‘pseudo-irreversible' antagonist, as it depressed the maximum response to 5-HT significantly (Figure 3). In other bioassays, SB 204070 was a ‘pseudo-irreversible' antagonist as well (Wardle et al., 1994; Leung et al., 1996; Zeitung et al., 1998) and similar apparent pA2 values, varying between 10 and 11, were found, being in good accordance with the affinity estimate obtained in canine tissue (10.6) in the current study. The selective 5-HT4 receptor antagonist RS 39604 (Hegde et al., 1995; Figure 3b) competitively antagonized the relaxations to 5-HT, yielding a pKB estimate (7.9) that was remarkably lower than that observed in the rat oesophagus tunica muscularis mucosae (pA2 9.3; Hegde et al., 1995; Table 2). This deviation in affinity for RS 39604 could be due to structural heterogeneity among 5-HT4 receptors in various mammals. Alternatively, a number of publications have mentioned splice variants of the 5-HT4 receptor being expressed in rats (Gerald et al., 1995), pigs (Ullmer et al., 1995) and humans (Van den Wyngaert et al., 1997; Blondel et al., 1998), which could result in pharmacological differences among tissues within animal species. However, as no evidence has yet emerged of 5-HT4 receptor splice variants being expressed in the dog, the affinity estimate for RS 39604 being divergent to this extent is most likely explained by the rat and canine 5-HT4 receptor being structurally different. Furthermore, as GR 113808, GR 125487 and SB 204070 do not express such a divergent affinity (Table 2), it seems that only RS 39604 might pharmacologically distinguish between 5-HT4 receptors of the dog and of other animal species. Still, the overall profile of the obtained antagonist affinity estimates ultimately point to 5-HT4 receptors mediating the relaxation to 5-HT. This point is further emphasized by the agonist-independent affinity estimate of GR 113808, using 5-HT and R076186 as an agonist (Figure 4).
The findings of this study are in line with previous observations by Briejer and colleagues (1998a), who investigated motility patterns in conscious dogs that had been equipped with chronically implanted, circularly-placed, force transducers on the serosal side of the canine large intestine. They showed that the selective 5-HT4 receptor agonist prucalopride enhanced the motility in the proximal colon and inhibited the motility in the distal colon. This effect could be blocked by the selective 5-HT4 receptor antagonist GR 125487, indicative of 5-HT4 receptors mediating the changes in motility patterns. Accordingly, the present study revealed that the 5-HT-induced relaxation was relatively most pronounced in the rectum, and decreased using descending colon, or ascending colon, respectively. Therefore, it could be hypothesized that 5-HT4 receptor density and/or coupling increases towards the distal end of the large intestine.
Earlier, canine 5-HT4 receptor-mediated responses were found in the stomach using the Heidenhain pouch (i.e. focused on the proximal gastric area), as the highly potent and selective 5-HT4 receptor antagonist SB 204070 (Wardle et al., 1994) inhibited 5-HT-evoked increments in pressure in vivo (Bingham et al., 1995). However, in in vitro studies using the canine antrum (i.e. the distal gastric area), it was shown that the non-selective 5-HT4 receptor agonists cisapride and 5-HT facilitated the cholinergic neurotransmission in the canine antrum in vitro, an effect that was insensitive to 5-HT4 receptor blockade (de Ridder & Schuurkes, 1993). Therefore, the link between the in vitro and the in vivo data, as is proposed for the canine large intestine, has not yet been established for the stomach.
To date, most studies on 5-HT4 receptors have been performed on other species than the dog. In the guinea-pig, 5-HT4 receptors are located exclusively on the enteric neurones, facilitating cholinergic and tachykininergic neurotransmission, resulting in contraction (Briejer & Schuurkes, 1996). In the rat oesophagus tunica muscularis mucosae preparation (Baxter et al., 1991) and in the rat ileum (Tuladhar et al., 1996), smooth muscle 5-HT4 receptors mediate relaxation. 5-HT4 receptor stimulation in the rat colon has not yet been associated with contraction or relaxation, but with secretion (Bunce et al., 1991). However, in the human colon large intestine (ascending to sigmoid colon and rectum), circular smooth muscle 5-HT4 receptors mediate relaxation (Tam et al., 1995; Meulemans et al., 1995; McLean et al., 1995). Recently, we showed that the selective 5-HT4 receptor agonists prucalopride and R076186 mediate relaxation of the human colon, consistent with 5-HT4 receptor interactions (Prins et al., 1998b). These agonists also potently relaxed the canine rectum in the present study. Therefore, the dog may be the first animal species which resembles humans with respect to pharmacology and function of colonic 5-HT4 receptors. The canine rectum may be designated a well-predictive pharmacological model for the human colon concerning 5-HT4 receptor function.
To summarize, in this study it was established that 5-HT4 receptors on the canine rectal circular smooth muscle mediate relaxation. The observed affinity estimate of RS 39604, which is lower than previously reported using rat tissue, might indicate that the canine 5-HT4 receptor is pharmacologically distinguishable from the 5-HT4 receptor of the rat. In conclusion, the canine rectum provides a 5-HT4 receptor model for the human colon.
Acknowledgments
The authors wish to thank André Van De Water and Henk van der Linde from the Department of Cardiovascular & Pulmonary Pharmacology of the Janssen Research Foundation for providing canine tissue.
Abbreviations
- 5-MeOT
5-methoxytryptamine
- MCh
methacholine
References
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. Chemother. 1959;14:48–54. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAXTER G.S., CRAIG D.A., CLARKE D.E. 5-Hydroxytryptamine4 receptors mediate relaxation of the rat oesophageal tunica muscularis mucosae. Naunyn-Schmiedebergs' Arch. Pharmacol. 1991;343:439–446. doi: 10.1007/BF00169544. [DOI] [PubMed] [Google Scholar]
- BINGHAM S., KING B.F., RUSHANT B., SMITH M.I., GASTER L., SANGER G.J. Antagonism by SB 204070 of 5-HT-evoked contractions in the dog stomach: an in-vivo model of 5-HT4 receptor function. J. Pharm. Pharmacol. 1995;47:219–222. doi: 10.1111/j.2042-7158.1995.tb05782.x. [DOI] [PubMed] [Google Scholar]
- BLACK J.W., LEFF P., SHANKLEY N.P. Further analysis of anomalous pKB values for histamine H2-receptor antagonists on the mouse isolated stomach assay. Br. J. Pharmacol. 1985;86:581–587. doi: 10.1111/j.1476-5381.1985.tb08934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLONDEL O., GASTINEAU M., DAHMOUNE Y., LANGLOIS M., FISCHMEISTER R. Cloning, expression, and pharmacology of four human 5-hydroxytryptamine4 receptor isoforms produced by alternative splicing in the carboxyl terminus. J. Neurochem. 1998;70:2252–2261. doi: 10.1046/j.1471-4159.1998.70062252.x. [DOI] [PubMed] [Google Scholar]
- BRIEJER M.R., AKKERMANS L.M.A., MEULEMANS A.L., LEFEBVRE R.A., SCHUURKES J.A.J. Cisapride and a structural analogue, R 76,186, are 5-hydroxytryptamine4 (5-HT4) receptor agonists on the guinea-pig colon ascendens. Naunyn-Schmiedebergs' Arch. Pharmacol. 1993;347:464–470. doi: 10.1007/BF00166736. [DOI] [PubMed] [Google Scholar]
- BRIEJER M.R., AKKERMANS L.M.A., SCHUURKES J.A.J. Gastrointestinal prokinetic benzamides: the pharmacology underlying stimulation of motility. Pharmacol. Rev. 1995;47:631–651. [PubMed] [Google Scholar]
- BRIEJER M.R., GHOOS E., EELEN J., SCHUURKES J.A.J. Serotonin 5-HT4 receptors mediate the R093877-induced changes in contractile patterns in the canine colon. Gastroenterology. 1998a;112:A705. [Google Scholar]
- BRIEJER M.R., MEULEMANS A.L., BOSMANS J.-P., VAN DAELE P., SCHUURKES J.A.J. In vitro pharmacology of the novel enterokinetic R093877. Gastroenterology. 1998b;112:A704. [Google Scholar]
- BRIEJER M.R., SCHUURKES J.A.J. 5-HT3 and 5-HT4 receptors and cholinergic and tachykininergic neurotransmission in the guinea-pig proximal colon. Eur. J. Pharmacol. 1996;308:173–180. doi: 10.1016/0014-2999(96)00297-x. [DOI] [PubMed] [Google Scholar]
- BUCHHEIT K.H., GAMSE R., GIGER R., HOYER D., KLEIN F., KLOPPNER E., PFANNKUCHE H.J., MATTES H. The serotonin 5-HT4 receptor. 1. Design of a new class of agonists and receptor map of the agonist recognition site. J. Med. Chem. 1995;38:2326–2330. doi: 10.1021/jm00013a009. [DOI] [PubMed] [Google Scholar]
- BUNCE K.T., ELSWOOD C.J., BALL M.T. Investigation of the 5-hydroxytryptamine receptor mechanism mediating the short-circuit current response in rat colon. Br. J. Pharmacol. 1991;102:811–816. doi: 10.1111/j.1476-5381.1991.tb12257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE RIDDER W.J., SCHUURKES J.A.J. Cisapride and 5-hydroxytryptamine enhance motility in the canine antrum via separate pathways, not involving 5-hydroxytryptamine1,2,3,4 receptors. J. Pharmacol. Exp. Ther. 1993;264:79–88. [PubMed] [Google Scholar]
- GALE J.D., GROSSMAN C., DARTON J., BUNCE K.T., WHITEHEAD J.W.F., KNIGHT J., PARKHOUSE T.J., OXFORD A.W., HUMPHREY P.P.A. GR125487: A selective and high affinity 5-HT4 receptor antagonist. Br. J. Pharmacol. 1994a;113 suppl.:120P. doi: 10.1111/j.1476-5381.1994.tb14064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALE J.D., GROSSMAN C.J., WHITEHEAD J.W., OXFORD A.W., BUNCE K.T., HUMPHREY P.P. GR113808: a novel, selective antagonist with high affinity at the 5-HT4 receptor. Br. J. Pharmacol. 1994b;111:332–338. doi: 10.1111/j.1476-5381.1994.tb14064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERALD C., ADHAM N., KAO H.T., OLSEN M.A., LAZ T.M., SCHECHTER L.E., BARD J.A., VAYSSE P.J., HARTIG P.R., BRANCHEK T.A. The 5-HT4 receptor: molecular cloning and pharmacological characterization of two splice variants. EMBO Journal. 1995;14:2806–2815. doi: 10.1002/j.1460-2075.1995.tb07280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOMMEREN W., RENDERS J., VAN GOMPEL P., LESAGE A., LEYSEN J., JURZAK M. Extensive pharmacological study of the G protein-coupled fraction of human 5-HT receptors using agonist radioligand binding. Naunyn-Schmiedebergs' Arch. Pharmacol. 1998;358:P8.42. [Google Scholar]
- GRIDER J.R., FOXX-ORENSTEIN A.E., JIN J.G. 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea-pig intestine. Gastroenterology. 1998;115:370–380. doi: 10.1016/s0016-5085(98)70203-3. [DOI] [PubMed] [Google Scholar]
- HEGDE S.S., BONHAUS D.W., JOHNSON L.G., LEUNG E., CLARK R.D., EGLEN R.M. RS 39604: a potent, selective and orally active 5-HT4 receptor antagonist. Br. J. Pharmacol. 1995;115:1087–1095. doi: 10.1111/j.1476-5381.1995.tb15922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEGDE S.S., EGLEN R.M. Peripheral 5-HT4 receptors. FASEB. 1996;10:1398–1407. doi: 10.1096/fasebj.10.12.8903510. [DOI] [PubMed] [Google Scholar]
- LEUNG E., PULIDO-RIOS M.T., BONHAUS D.W., PEKINS L.A., ZEITUNG K.D., HSU S.A., CLARK R.D., WONG E.H., EGLEN R.M. Comparison of 5-HT4 receptors in guinea-pig colon and rat oesophagus: effects of novel agonists and antagonists. Naunyn-Schmiedebergs' Arch. Pharmacol. 1996;354:145–156. doi: 10.1007/BF00178714. [DOI] [PubMed] [Google Scholar]
- MCLEAN P.G., COUPAR I.M., MOLENAAR P. A comparative study of functional 5-HT4 receptors in human colon, rat oesophagus and rat ileum. Br. J. Pharmacol. 1995;115:47–56. doi: 10.1111/j.1476-5381.1995.tb16318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEULEMANS A.L., GHOOS E., CHEYNS P., SCHUURKES J.A.J. 5-HT-induced relaxations of human sigmoid colon are mediated via 5-HT4 receptors. Pfluger's Arch. Eur. J. Physiol. 1995;429:R9. [Google Scholar]
- NAGAKURA Y., KAMATO T., NISHIDA A., ITO H., YAMANO M., MIYATA K. Characterization of 5-hydroxytryptamine (5-HT) receptor subtypes influencing colonic motility in conscious dogs. Naunyn-Schmiedebergs' Arch. Pharmacol. 1996;353:489–498. doi: 10.1007/BF00169167. [DOI] [PubMed] [Google Scholar]
- PRINS N.H., BRIEJER M.R., SCHUURKES J.A.J. Characterization of the contraction to 5-HT in the canine colon longitudinal muscle. Br. J. Pharmacol. 1997;120:714–720. doi: 10.1038/sj.bjp.0700954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRINS N.H., BRIEJER M.R., SCHUURKES J.A.J., AKKERMANS L.M.A. Contractile effects of carbachol and 5-HT in the canine colon circular muscle. Naunyn-Schmiedebergs' Arch. Pharmacol. 1998a;358 suppl.:P40.21. [Google Scholar]
- PRINS N.H., BRIEJER M.R., SCHUURKES J.A.J., AKKERMANS L.M.A. Optimisation of a 5-HT4 receptor bioassay in human isolated colon circular muscle. Neurogastroenterol. Motility. 1998b;10:478. [Google Scholar]
- SANGER G.J. 5-Hydroxytryptamine and functional bowel disorders. Neurogastroenterol. Motility. 1996;8:319–331. doi: 10.1111/j.1365-2982.1996.tb00270.x. [DOI] [PubMed] [Google Scholar]
- TAM F.S., HILLIER K., BUNCE K.T., GROSSMAN C. Differences in response to 5-HT4 receptor agonists and antagonists of the 5-HT4-like receptor in human colon circular smooth muscle. Br. J. Pharmacol. 1995;115:172–176. doi: 10.1111/j.1476-5381.1995.tb16335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TULADHAR B.R., COSTALL B., NAYLOR R.J. Pharmacological characterization of the 5-hydroxytryptamine receptor mediating relaxation in the rat isolated ileum. Br. J. Pharmacol. 1996;119:303–310. doi: 10.1111/j.1476-5381.1996.tb15986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ULLMER C., SCHMUCK K., KALKMAN H.O., LUBBERT H. Expression of serotonin receptor mRNAs in blood vessels. FEBS. 1995;370:215–221. doi: 10.1016/0014-5793(95)00828-w. [DOI] [PubMed] [Google Scholar]
- VAN DEN WYNGAERT I., GOMMEREN W., VERHASSELT P., JURZAK M., LEYSEN J., LUYTEN W., BENDER E. Cloning and expression of a human serotonin 5-HT4 receptor cDNA. J. Neurochem. 1997;69:1810–1819. doi: 10.1046/j.1471-4159.1997.69051810.x. [DOI] [PubMed] [Google Scholar]
- WARDLE K.A., ELLIS E.S., BAXTER G.S., KENNETT G.A., GASTER L.M., SANGER G.J. The effects of SB 204070, a highly potent and selective 5-HT4 receptor antagonist, on guinea-pig distal colon. Br. J. Pharmacol. 1994;112:789–794. doi: 10.1111/j.1476-5381.1994.tb13148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZEITUNG K.D., PERKINS L.A., HSU S., BONHAUS D., EGLEN R.M., WONG A.G., LEUNG E. Comparison of GR 113808 and SB 204070 at 5-HT4 receptors in vitro. FASEB. 1998;8:A92–533. [Google Scholar]


