Abstract
Moderate consumption of red wine has been associated with beneficial effects on human health, and this has been attributed to the flavonoid content. Factors that influence the bioavailability of this group of polyphenolic compounds are therefore important.
Using the rat cannulated everted jejunal sac technique, we have investigated the effect of alcohol on the intestinal absorption of quercetin and its 3-O-glucoside from red wine. Tissue preparations were incubated in whole or dealcoholised red wine, diluted 1 : 1 with Krebs buffer for 20 min at 37°C, after which the mucosa was removed and processed for HPLC analysis. Tissues exposed to red wine had significantly higher amounts of both quercetin (× 3; P<0.001) and quercetin-3-O-glucoside (× 1.5; P<0.01) associated with them, compared with sacs incubated in the dealcoholised equivalent. In addition, both tamarixetin (T) and isorhamnetin (I), in the mucosal tissue from sacs exposed to the whole wine, were significantly elevated approximately two fold (P<0.05; P<0.01, respectively).
Similar results were obtained when sacs were incubated in Krebs buffer containing a mixture of pure quercetin and quercetin-3-O-glucoside with or without alcohol, and, although effects on the apparent absorption of Q and Q-3-G were not so marked, concentrations of the metabolites quercetin-3-O-glucuronide and I were significantly increased by the presence of alcohol (P<0.01 and P<0.001, respectively).
It is therefore plausible that the moderate alcohol content of red wine contributes to its beneficial health effects in humans by both increasing the absorption of quercetin and quercetin-3-O-glucoside and by channelling their metabolism towards O-methylation to yield compounds (T and I), which have potential protective effects against cancer and cardiovascular diseases.
Keywords: Red wine, quercetin, ethyl alcohol, intestinal absorption, quercetin metabolism
Introduction
It is widely accepted that a diet rich in vegetables and including a moderate intake of wine is associated with a lower incidence of cardiovascular disease and cancer. These protective effects may be due to the presence of flavonoids, a broad class of low molecular weight compounds characterised by a flavan nucleus (e.g. rutin, quercetin (Q), myricetin, oleoeuropeine, resveratrol). Although it is generally believed that the health effects of flavonoids are dependent on their bioavailability, they also have potential beneficial effects in the gastric lumen, where, by inhibiting lipid oxidation, they prevent the formation of harmful radicals from food components (Kanner & Lapidot, 2001). Q, the major flavonol in the plant kingdom, is a powerful scavenger of free radicals and is also able to interact with endogenous proteins (Kaldas et al., 2005). Q, although inducing both endothelium-dependent (Fusi et al., 2003) and endothelium-independent vasorelaxation in vitro (Duarte et al., 1993; Herrera et al., 1996), has been demonstrated to be an effective stimulator of vascular smooth muscle L-type Ca2+ channels (Saponara et al., 2002) in a manner similar to that of its structural analogue myricetin (Fusi et al., 2005). Although the pharmacological properties of Q have been thoroughly investigated, very little information is available as to the activity of its conjugated forms, which are predominant in foods (Murota & Terao, 2003). Recent studies indicating that Q can be absorbed by humans from the diet as glycosides (Hakkinen & Auriola, 1998) have stimulated an interest in studying the bioavailability of flavonol glycosides from various dietary sources. There is evidence to suggest that Q glucosides interact with the small intestinal sodium-dependent glucose transporter (SGLT1) (Gee et al., 1998; 2000), or are deglycosylated at the mucosal surface by lactase phlorizin hydrolase (LPH) (Day et al., 2003), the resulting aglycone entering the cell by diffusion.
Epidemiological studies have overwhelmingly supported the notion that moderate alcohol consumption lowers the risks of mortality, hospitalisation and early clinical symptoms of coronary artery disease as compared to abstinence from alcohol consumption (Soleas et al., 2001). Moreover, in a recent study Mukamal et al. (2005) pointed out that whereas moderate red wine consumption has an inverse association with ischaemic stroke, other alcoholic beverages do not. This could be because of the high polyphenolic antioxidant content of the wine, compared to that of other alcoholic beverages. It has been demonstrated that red wine, but not its dealcoholised counterpart, increases brachial artery diameter in human volunteers at rest (Agewall et al., 2000), and induces favourable changes in serum lipoproteins of healthy young men (Koga & Meydani, 2001). There has been some speculation that the presence of alcohol in red wine might improve flavonoid availability by increasing its intestinal absorption, or by delaying its excretion (Ruf et al., 1995). Data on the intestinal absorption of flavonols from wine are sparse (Goldberg et al., 2003), and it is not yet known to what extent these components are bioavailable, and whether alcohol plays a role in their absorption.
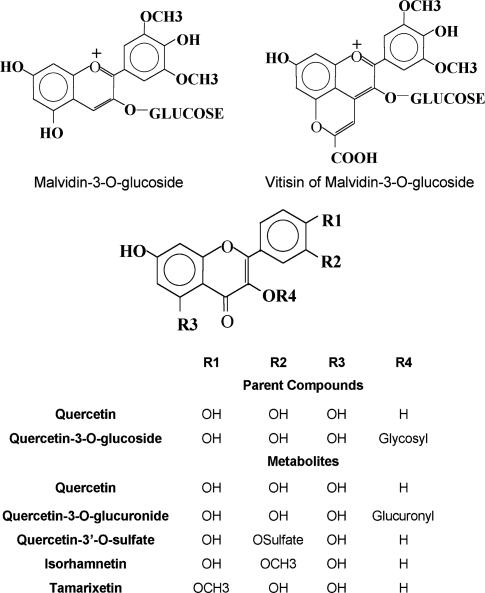
To obtain a better understanding of the effects of alcohol on the intestinal absorption of Q and quercetin-3-O-glucoside (Q-3-Glc) (Figure 1) present in red wine, cannulated everted rat jejunal sacs were exposed to whole red wine, a dealcoholised product obtained by freeze-drying and a synthetic mixture of the aglycone and glycoside with and without alcohol.
Figure 1.
Structure of some naturally occurring flavonoids.
Methods
Whole wine and dealcoholised wine
The red wine used for the experiment was Rosso di Montalcino, vintage 2002, kindly provided by Banfi srl, Montalcino, Siena, Italy. Red wine samples (40 ml) in glass cylinders were connected to a freeze-drying apparatus (BOC Edwards, Tonawanda, NY, U.S.A.) and freeze-dried under vacuum (100 mtorr). Water was then added to restore the sample volume to 40 ml. The whole and dealcoholised wine samples were diluted 1 : 1 with double strength Krebs buffer to reduce the level of alcohol and mimic those reaching the small bowel (Levitt et al., 1997), and to achieve a chemical composition and pH values as close as possible to physiological conditions, with some buffering capacity – both solutions had good clarity and there was no apparent, undissolved material – present in either one.
Determination of volatile acidity and sulphur dioxide
Quantitative analyses for volatile acids and sulphur dioxide were performed according to official methods (ECC commission regulation VO 2676/90).
Determination of antioxidant activity of whole and dealcoholised red wines
The total antioxidant activity of full and dealcoholised wine samples was evaluated, after dilution 1 : 100, according to Pellegrini et al. (2001). Results were expressed as trolox equivalent antioxidant capacity (TEAC) and given as mmol l−1.
Determination of phenols
Whole and dealcoholised wine samples were diluted 1 : 100 and their phenolic content was determined by the Folin—Ciocalteu method (Singleton & Rossi, 1965). Results of duplicate analyses are expressed as milligrams of gallic acid equivalents (GAE) per liter.
Animals
Male Wistar rats (∼200 g) were obtained from a licensed commercial animal supplier and housed in an environmentally controlled animal facility prior to use. All aspects of animal care complied with the ethical guidelines and technical requirements of the U.K. Home Office.
Intestinal preparation
Each rat was deeply anaesthetised with pentobarbital (Euthetal; Rhone Merieux, Harlow, Essex, U.K.) and killed by cervical dislocation immediately before the removal of the small intestine via an abdominal incision. The jejunum was identified (10–50% length), rinsed with 20 ml Krebs bicarbonate buffer (118 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 24.4 mM NaHCO3 in distilled water, gassed with oxygen : carbon dioxide 95 : 5 to give a final pH 7.2–7.4) and everted. Four segments (5 cm) were cut from the proximal portion and incubated as described in Tables 1 and 2. Sacs were prepared as described in Gee et al. (1998).
Table 1.
Flavonols and anthocyanins, determined by HPLC, in mucosal scrape samples of everted rat jejunal sacs incubated with whole and dealcoholised wine solutions
| Incubation solution | ||
|---|---|---|
| Analyte | Whole wine (pmol/g w.t.) | Dealcoholised wine (pmol/g w.t.) |
| Q | 17,532±1493*** | 5907±464 |
| Q-3-Glc | 9533±835** | 6107±468 |
| M-3-G | 4006±694 | 2650±356 |
| PA-AD | 644±131 | 537±62 |
| Q-3-O-GLA | 260±39 | 735±158* |
| Q-3′-O-S | ND | 1242±168 |
| Ta | 1237±69** | 561±188 |
| I | 1525±91* | 889±172 |
Expressed as pmol of isorhamnetin.
Cannulated everted sacs were prepared by ligaturing everted segments at one end and tying the other ends to tapered disposable syringes (1 ml), precharged with Krebs bicarbonate buffer (0.5 ml, pH 7.4). The sacs were suspended in organ baths (37°C) containing incubation media (8 ml) as described below, and gassed continuously with 5% carbon dioxide in oxygen for 20 min.
For each rat (n=5), sacs were incubated in diluted rosso di Montalcino wine (final pH, 4.8) or diluted dealcoholised rosso di Montalcino wine (final pH, 4.8). Data normalised for recovery and mass (g). Figures represent mean values±s.e.m. from five animals.
ND=not detectable.
P<0.05;
P<0.01;
P<0.001 compared to dealcoholised wine.
Table 2.
Flavonols and anthocyanins, determined by HPLC, in mucosal scrape samples of everted rat jejunal sacs incubated with Q plus Q-3-G solution in the presence or absence of ethanol
| Analyte | Incubation solution | |
|---|---|---|
| Q+Q-3-Glc+ethanol (pmol/g w.t.) | Q+Q-3-Glc (pmol/g w.t.) | |
| Q | 5946±626** | 3404±392 |
| Q-3-Glc | 637±45 | 705±74 |
| M-3-G | ND | ND |
| PA-AD | ND | ND |
| Q-3-O-GLA | 1266±122** | 432±61 |
| Q-3′-O-S | 1483±274 | 1210±171 |
| Ta | ND | ND |
| I | 1556±84*** | 609±81 |
Expressed as pmol of isorhamnetin.
Four segments (5 cm) were cut from the proximal portion and incubated, as described in Table 1, in Krebs buffer containing quercetin (15 μM) and quercetin-3-glucoside (30 μM) with absolute ethanol (6.5% vv−1) (final pH, 7.3), or in the same quercetin–quercetin glucoside solution without alcohol.
Data normalised for recovery and mass (g). Figures represent mean values±s.e.m. from five animals.
ND=not detectable.
*P<0.05;
P<0.01;
P<0.001 compared to dealcoholised wine.
At the end of the incubation period, the serosal solutions were removed. Sacs were cut from the cannulae and the mucosal tissue was gently scraped from each sac. This was then transferred to Eppendorf tubes and immediately snap-frozen on dry ice. Each mucosal solution was also sampled at the end of the incubation period. Serosal and mucosal solutions and samples of the initial mucosal solutions that had not been used in the incubation were immediately extracted as follows. Mucosal tissue was stored at −20°C before the extraction.
Preparation of samples
A total of 400 μl of serosal or mucosal solution was transferred to an Eppendorf tube containing 7 μl concentrated HCl and 50 μl 0.1 mg ml−1 naringin (internal standard (IS)) in methanol. After mixing, the samples were centrifuged (14,000 × g, g, 5 min, 4°C), and the supernatant transferred to vial before HPLC.
Each mucosal scrape was weighed while frozen, and spiked with 50 μl 0.1 mg ml−1 naringin as an IS and homogenised with methanol (0.25 ml) containing ascorbic acid (1 mM) to stabilise the samples during analysis. Following centrifugation (14,000 × g, g, 5 min, 4°C), the supernatant was removed and retained, and the pellet re-extracted with methanol/ascorbate solution as before. The supernatants were combined and concentrated to dryness under a stream of N2 gas. Methanol was added to give a final volume of 125 μl. Following a further centrifugation (14,000 × g, 4°C, 5 min), the supernatant was analysed by HPLC.
HPLC analysis
HPLC analysis was performed using an Agilent HP1100 binary system (Agilent Technologies, Waldbronn, Germany) equipped with a thermostatically controlled autosampler (set at 10°C), a thermostatically controlled column oven and a diode array detector (Hewlett-Packard, Palo Alto, CA, U.S.A.) set to collect data at 270 nm for most UV-absorbing compounds, 370 nm, to detect the presence of flavonoids; and 520 nm, for anthocyanins.
A Phenomenex Luna C18(2) reverse-phase column (250 × 4.6 mm, 5 μm) was used, in combination with a ‘Securityguard' C18 precolumn. The flow rate was 1 ml min−1, column temperature was 30°C and injection volume was 20 μl. Elution solvents were 0.1% (v v−1) TFA (trifluoroacetic acid; Sigma/Aldrich, Poole, Dorset, U.K.) in Milli Q water (A) and 0.1% (v v−1) TFA in HPLC grade acetonitrile (B). The linear gradient was 0 min (100% A, 0% B), 5 min (100% A, 0% B), 15 min (83% A, 17% B), 17 min (83% A, 17% B), 22 min (75% A, 25% B), 30 min (65% A, 35% B), 35 min (50% A, 50% B), 40 min (0% A, 100% B), 50 min (0% A, 100% B), 55 min (100% A, 0% B) and 65 min (100% A, 0% B).
Recovery of the IS naringin at 270 nm was 72 and 70% for mucosal scrape and serosal samples, respectively.
All mass spectra were obtained using a Micromass Quattro II triple quadrupole mass spectrometer (Micromass, Manchester, U.K.) coupled with a Jasco PU-1585 triple pump HPLC equipped with an AS-1559 cooled autoinjector, CO-1560 column oven and UV-1575 UV detector (Jasco (U.K.) Ltd, Great Dunmow, U.K.).
Mass spectra were obtained in both positive ion and negative ion electrospray modes using a Micromass Z-spray ion source. Spectra were processed using MassLynx 3.4 software (Micromass, Manchester, U.K.). All values are presented as mean values±standard error of the mean (s.e.m.). The significance of the differences between means from the two experimental groups was determined by using Student's t-test. P<0.05 was considered to be significant.
Results
Alcohol present in whole wine enhances intestinal absorption of Q and Q-3-G, and directs Q metabolism towards tamarixetin (T) and isorhamnetin (I), while depressing quercetin-3′-O-sulphate formation (Q-3′-O-S)
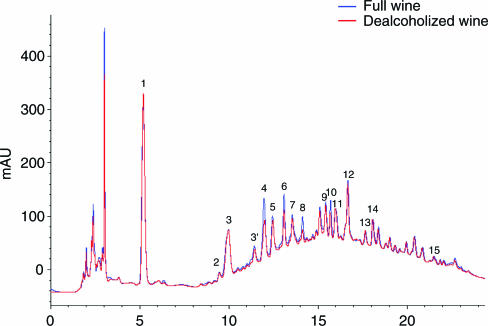
In whole and dealcoholised red wine samples, Q and Q-3-Glc concentrations, were comparable, averaging 7.4 and 5.4 μM (Q) and 5.4 and 5.2 μM (Q-3-Glc), respectively. During the freeze-drying procedure, the loss of Q and Q-3-Glc did not exceed 15 and 5%, respectively. The total polyphenol content and the antioxidant activity of whole and dealcoholised wine samples were comparable; the mean polyphenol content was 17.6 and 17.8 mM of GAE, and antioxidant capacity 27.2 and 26.8 mM of TEAC, respectively. In contrast, there was a substantial loss of volatile acidity and sulphur dioxide (SO2) in dealcoholised wine (75 mg l−1 of acetic acid equivalent compared with 450 mg l−1 in whole wine; 9 p.p.m. free and 27 p.p.m. total SO2 compared with 30 and 92 p.p.m. in whole wine). As shown in Figure 2, the HPLC profiles of both whole and dealcoholised wines were qualitatively and quantitatively virtually identical, with the exception of procyanidin B1 (4), catechin (6), procyanidin B2 (8) and epicatechin (10) in dealcoholised wine, for which peak areas were slightly reduced (max. 30%).
Figure 2.
HPLC chromatograms of full and dealcoholised wine monitored at 270 nm. 1: Gallic acid; 2: protocatechuic acid; 3: caftaric acid; 3′: procyanidin B3; 4: procyanidin B1; 5: not indentified; 6: catechin; 7: not indentified; 8: procyanidin B2; 9: caffeic acid; 10: epicatechin; 11: not indentified; 12: siringic acid; 13: procyanidin B4; 14: p-cumaric acid; 15: not identified.
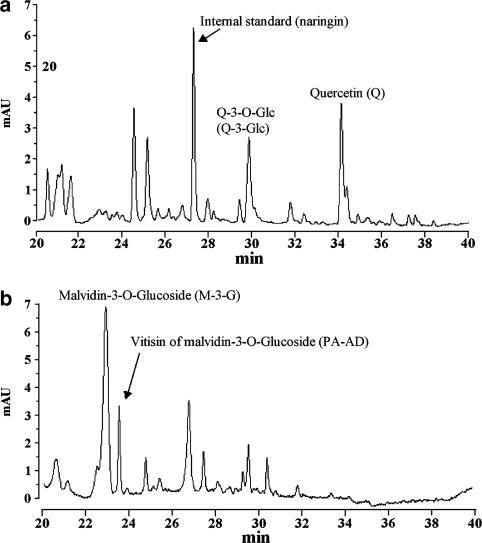
When everted jejunal sacs were incubated for 20 min at 37°C in solutions containing either whole wine or its dealcoholised derivative, consistent amounts of Q and Q metabolites were found in the mucosal tissue. However, under the same conditions, none of the analytes was detected in the serosal solutions. HPLC analysis of extracted samples of mucosal tissue revealed the presence of a complex range of flavonols (Figure 3a) and anthocyanins (Figure 3b).
Figure 3.
HPLC chromatograms of an extract of intestinal mucosal tissue following exposure to whole red wine. Absorbance of flavonols (a) was monitored at 270 nm. Absorbance of anthocyanins was recorded at 520 nm (b).
As shown in Table 1, there were significantly higher amounts of both Q (300%) and Q-3-Glc (150%) in tissue exposed to whole red wine solution than in tissue incubated with dealcoholised red wine. HPLC analysis of the tissues also revealed the presence of two additional compounds, characterised by retention times of 22.98 and 23.57 min, respectively, which were subsequently identified by electrospray mass spectrometry as malvidin-3-O-glucoside (M-3-G), one of the most abundant anthocyanins in red wine, and vitisin A (PA-AD), which is formed mostly through the condensation of M-3-G and pyruvic acid during red wine ageing (Garcia-Alonso et al., 2004). There was a tendency towards higher concentrations of both compounds in extracts of tissues incubated in whole red wine solution, although this did not reach significance. Under these experimental conditions, almost all tissue samples contained detectable amounts of the Q metabolites, quercetin-3-O-glucuronide (Q-3-O-GLA), Q-3′-O-S, T and I characterised by retention times of 26.1, 33.6, 37.4 and 37.8 min, respectively. Figure 1 illustrates the structure of the compounds.
Furthermore, after incubation of tissues with the whole wine solution, the amount of Q-3-O-GLA was significantly lower (40%) than that detected in tissues incubated with dealcoholised wine. Q-3′-O-S, was not present in detectable amounts after the incubation of tissues with whole wine solution, whereas in tissues incubated with dealcoholised wine solution, it was detected, and amounts averaged 206 pmol g−1. In contrast to these observations, the T and I contents of mucosal tissue from sacs incubated with whole red wine solution were approximately 220 and 170% higher, respectively, than those found in tissues incubated with the dealcoholised wine solution.
Ethyl alcohol present in a synthetic mixture of Q and Q-3-G enhances intestinal absorption of Q and directs its metabolism towards Q-3-O-GLA and I
As shown in Table 2, when the everted rat jejunal sacs were incubated with a synthetic mixture of Q and Q-3-Glc with or without alcohol, some of the observations with whole red wine and dealcoholised wine solutions were confirmed. In this case, the presence of alcohol gave rise to a significant increase in Q content in mucosal tissue (175%), whereas the amount of Q-3-Glc appeared unchanged. In contrast, with respect to the Q metabolites detected, there was a significantly higher amount of Q-3-O-GLA and I (300 and 250%, respectively) in the presence of alcohol than in sacs incubated with Q and Q-3-Glc without alcohol. However, levels of Q-3′-O-S in preparations incubated with either of these solutions did not vary significantly.
In contrast to the effects seen in the tissues, the bathing (mucosal) solutions, collected at the end of each incubation, did not contain detectable concentrations of any of the Q metabolites (Q-3-O-GLA, Q-3′-O-S, T, I). After 20 min incubation, however, the Q and Q-3-Glc content of the red wine solution decreased by about 10 and 40%, respectively, compared with initial values. Similarly, in the case of the dealcoholised wine, amounts of Q and Q-3-Glc both decreased by about 30%. This could not be accounted for in terms of tissue accumulation or serosal transfer, and it is assumed that the imbalance is because of chemical degradation in the gut lumen. Q is unstable at 37°C under aerobic conditions, breaking down to form equally unstable quinine methides and ring fission products due to further oxidation (Awad et al., 2001; 2003).
Interestingly enough, serosal samples also did not contain even traces of Q, Q-3-Glc and related metabolites. These negative findings suggest that Q metabolites once formed in the mucosa do not undergo either backward or forward diffusion in appreciable amounts into the mucosal or the serosal solution compartments, respectively.
Discussion
This study demonstrates that incubating rat jejunal sacs in vitro with whole red wine gives rise to significantly higher amounts of both Q and Q-3-Glc associated with the mucosal tissue compared with levels detected after incubation with dealcoholised wine. The fact that similar results with respect to Q uptake were obtained in modelled incubation mixtures suggests that the alcohol content is a major factor responsible for the higher amount of Q detected in mucosal tissue incubated with whole red wine. Although sacs were well rinsed in Krebs buffer before use, to remove luminal contamination, and in physiological saline after incubation, before removal of the mucosa, to minimise contamination with the bathing solution, it is possible that traces of Q and Q-3-G may have been trapped in the intervillous extracellular space. However, and particularly in the case of incubations with the Q plus Q-3-G solution, the relative proportions of these compounds associated with the mucosa (Q : Q-3-G, 9 : 1 with alcohol and 5 : 1 without alcohol; Table 2) are different from those of the bathing solution (1 : 2). The relative amounts extracted from mucosal tissue support the concept of deglycosylation of Q-3-G by LPH at the mucosal surface, and the entry of Q into the epithelial cells is reinforced by the presence of metabolites in significant quantities. Whereas it is entirely feasible that free Q exists in the tissue, the presence of Q-3-G could be disputed. However, it is more likely that the glucoside, rather than the aglycone, would bind to mucins, and the lack of any difference in ‘mucosal' Q-3-G between the two incubations with the synthetic mixture is indicative that either no binding occurred or that the alcohol had no effect on the degree of binding. In the case of incubations with the red wine solutions, a difference in Q-3-G concentration was seen, and it would therefore appear that uptake or metabolism of the glycoside by the mucosal epithelium, either intracellularly or paracellularly, is most likely facilitated by another component of red wine.
As reported previously (Gee et al., 2000), Q detected in the mucosal tissue may be the result of different mechanisms of transport, involving both the Q aglycone diffusing passively into enterocytes and Q-3-Glc being transported intact via the SGLT1, and subsequently deglycosylated by cytosolic β-glucosidases. Alternatively, Q-3-Glc may undergo extracellular hydrolysis by the brush-border enzyme LPH, the resulting aglycone passively diffusing into enterocytes. Ruf et al. (1995) speculated that the presence of alcohol in red wine may improve flavonoid bioavailability by increasing its intestinal absorption, however, they presented no experimental data to support this hypothesis. Ma et al. (1999), using CacO-2 cells, suggested that ethanol at low, noncytotoxic doses causes the opening of the CacO-2 cell tight-junction barrier by activating myosin light-chain kinase. Our observations may thus be explained by alcohol enhancing the permeability of the mucosal cell membrane, thus facilitating Q passive diffusion (Day et al., 2003) into the intestinal cells. Indeed, the facilitating effect of ethanol in the gastrointestinal absorption of some polar compounds in vivo (phenobarbitone, pentobarbitone, prometazine and sulphaguanidine) was reported by Magnussen (1968) some years ago, whereas in the stomach, ethanol enhanced the absorption of drugs through an increased blood supply to the gastric mucosa, in the small intestine, it had no clearcut effect on the absorption of the drugs tested. More recently, Bell et al. (2000) measured (+)-catechin concentration in human plasma after ingestion of whole red wine or the corresponding dealcoholised product, but saw no differences. They attributed this negative observation to the fact that the dealcoholisation procedure they used (rotatory evaporation under vacuum) invariably yields a product, whose chemical composition differs markedly from the original wine (Fisher et al., 1996). In the present study, the dealcoholised product, obtained by freeze-drying, was found to qualitatively and quantitatively match the original wine both, with respect to total polyphenol content. In addition, HPLC profiles were essentially identical both qualitatively and quantitatively, with very few exceptions. Indeed, the slightly lower amounts of procyanidin B1, catechin, procyanidin B2 and epicatechin in dealcoholised samples would, if anything, improve the absorption of Q-3-G, since it has been demonstrated that catechin and other polyphenols with the catechin structure play a role in the inhibition of rat intestinal SGLT1 (Kobayashi et al., 2000). Nevertheless, the one contrast between the two wine solutions remains the ethanol content, thus enabling the study reported here to focus on the influence of alcohol in the intestinal absorption of flavonols in wine.
Moreover, in the case of the synthetic mixture of Q and Q-3-Glc, the effect of alcohol on the intestinal uptake of Q was similar to that of red wine. However, as concentrations of the absorbed glycoside were similar, irrespective of the presence or absence of ethanol, the latter does not appear to be the sole cause of facilitated Q-3-Glc uptake from the intestinal lumen. It is, therefore, reasonable to assume that some yet undefined compounds present in red wine, but not in the dealcoholised counterpart, are responsible for the enhanced uptake of Q-3-Glc from whole wine.
Mucosal concentrations of major Q metabolites from whole and dealcoholised red wines suggest that ethanol is also responsible for the different metabolic processing of the parent compounds under the two different experimental conditions. The presence of alcohol appears to favour the pathway giving rise to the formation of T and I at the expense of the formation of both Q-3-O-GLA and Q-3′-O-S. Contrasting results were, however, obtained when incubating tissue with the synthetic mixture of Q plus Q-3-Glc in the presence or absence of alcohol. In the latter case, higher amounts of both Q-3-O-GLA and I were detected in the presence of alcohol, whereas the level of Q-3′-O-S was unaffected by ethanol. This can again be explained by the relative simplicity of this incubation mixture compared with the wines.
Other authors have demonstrated that complex interactions can take place between components of red wine, modulating some phase II enzymes in different ways. For example, whereas vanillic acid, caffeic acid and Q are potent inhibitors, in this respect, gallic, gentisic and p-hydroxybenzoic acids enhance phenolsulfotransferase activity in human platelets (Yeh & Yen, 2003). All these compounds occur naturally in red wine, and may thus interact with ethanol and contribute to the modulation of phase II metabolism of flavonoids. In contrast, such interactions could not have occurred in the incubations with the synthetic flavonoid mixture, where such components are absent. Previous reports have questioned the validity of using ethanol to mimic the effects of alcoholic beverages on intestinal permeability, as its effect, in isolation, on transport mechanisms may be different (Lemos et al., 2005).
The similar amounts of both M-3-G and PA-AD detected in mucosal tissue after incubation with whole wine and dealcoholised wine are in agreement with plasma levels in humans, following ingestion of whole and dealcoholised red wine, where plasma M-3-G response (area under the curve) did not differ (Bub et al., 2001). These data support the use of the everted intestinal sac preparation as a model to investigate further the complex series of events occurring during red wine uptake and metabolism. In conclusion, the present findings clearly show, for the first time, that ethyl alcohol plays a facilitating role in the small intestinal absorption of Q. Further experiments will clarify the mechanism(s) involved. Meanwhile, the observations allow us to speculate that the modest alcohol content in red wine contributes to its beneficial health effects, by both increasing the absorption of Q and Q-3-Glc, and by stimulating the formation of I and T, O-methylated metabolites of Q, compounds purported to be associated with the protective effects of Q against cancer and cardiovascular diseases (Jones et al., 2004). Further work would be necessary to confirm that the effect seen in this rat model can be reproduced in humans.
Acknowledgments
The study was supported by Fondazione Monte dei Paschi di Siena, COST ACTION 926 and the University of Siena. The assistance provided by Dr Elena Dreassi in some HPLC-MS analyses is gratefully acknowledged.
Abbreviations
- GAE
gallic acid equivalents
- I
isorhamnetin
- IS
internal standard
- LPH
lactase phlorizin hydrolase
- M-3-G
malvidin-3-O-glucoside
- PA-AD
vitisin A
- Q
quercetin
- Q-3-Glc
quercetin-3-O-glucoside
- Q-3-O-GLA
quercetin-3-O-glucuronide
- Q-3′-O-S
quercetin-3′-O-sulphate
- SGLT1
sodium-dependent glucose transporter
- T
tamarixetin
- TEAC
trolox equivalent antioxidant capacity
References
- AGEWALL S., WRIGHT S., DOUGHTY R.N., WHALLEY G.A., DUXBURY M., SHARPE N. Does a glass of red wine improve endothelial function. Eur. Heart J. 2000;21:74–78. doi: 10.1053/euhj.1999.1759. [DOI] [PubMed] [Google Scholar]
- AWAD H.M., BOERSMA M.G., BOEREN S., VAN BLADEREN P.J., VERVOORT J., RIETJENS I.M. Quenching of quercetin quinone/quinone methides by different thiolate scavengers: stability and reversibility of conjugate formation. Chem. Res. Toxicol. 2003;16:822–831. doi: 10.1021/tx020079g. [DOI] [PubMed] [Google Scholar]
- AWAD H.M., BOERSMA M.G., BOEREN S., VAN BLADEREN P.J., VERVOORT J., RIETJENS I.M. Structure–activity study on the quinone/quinone methide chemistry of flavonoids. Chem. Res. Toxicol. 2001;14:398–408. doi: 10.1021/tx000216e. [DOI] [PubMed] [Google Scholar]
- BELL J.R., DONOVAN J.L., WONG R., WATERHOUSE A.L., GERMAN J.B., WALZEM R.L., KASIM-KARAKAS S.E. (+)-Catechin in human plasma after ingestion of a single serving of reconstituted red wine. Am. J. Clin. Nutr. 2000;71:103–108. doi: 10.1093/ajcn/71.1.103. [DOI] [PubMed] [Google Scholar]
- BUB A., WATZL B., HEEB D., RECHKEMMER G., BRIVIBA K. Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, dealcoholized red wine and red grape juice. Eur. J. Nutr. 2001;40:113–120. doi: 10.1007/s003940170011. [DOI] [PubMed] [Google Scholar]
- DAY A.J., GEE J.M., DUPONT M.S., JOHNSON I.T., WILLIAMSON G. Absorption of quercetin-3-glucoside and quercetin-4′-glucoside in the rat small intestine: the role of lactase phlorizin hydrolase and the sodium-dependent glucose transporter. Biochem. Pharmacol. 2003;65:1199–1206. doi: 10.1016/s0006-2952(03)00039-x. [DOI] [PubMed] [Google Scholar]
- DUARTE J., PEREZ-VIZCAINO F., ZARZUELO A., JIMENEZ J., TAMARGO J. Vasodilator effects of quercetin in isolated rat vascular smooth muscle. Eur. J. Pharmacol. 1993;239:1–7. doi: 10.1016/0014-2999(93)90968-n. [DOI] [PubMed] [Google Scholar]
- ECC ECC Commission Regulation VO 2676/90 concerning the establishment of common analytical methods in the sector of wine. Offic. J. Eur. Communities. 1990;3:1–192. [Google Scholar]
- FISHER U., BERGER R.G., HAKANSSON A., NOBEL A.C.The impact of dealcoholization on the flavor of wine—relating concentration of aroma compounds to sensory data using PLS analysis Flavour Science: Recent Developments 1996Cambridge, U.K.: Royal Science of Chemistry; 335–338.eds. Taylor, A.J. & Mottram, D.S., pp8th Weurman Flavour Research Symposium [Google Scholar]
- FUSI F., SAPONARA S., PESSINA F., GORELLI B., SGARAGLI G. Effects of quercetin and rutin on vascular preparations: a comparison between mechanical and electrophysiological phenomena. Eur. J. Nutr. 2003;42:10–17. doi: 10.1007/s00394-003-0395-5. [DOI] [PubMed] [Google Scholar]
- FUSI F., SGARAGLI G., SAPONARA S. Mechanism of myricetin stimulation of vascular L-type Ca2+ current. J. Pharmacol. Exp. Ther. 2005;313:790–797. doi: 10.1124/jpet.104.080135. [DOI] [PubMed] [Google Scholar]
- GARCIA-ALONSO M., RIMBACH G., RIVAS-GONZALO J.C., DE PASCUAL-TERESA S. Antioxidant and cellular activities of anthocyanins and their corresponding vitisins A—studies in platelets, monocytes, and human endothelial cells. J. Agric. Food Chem. 2004;52:3378–3384. doi: 10.1021/jf035360v. [DOI] [PubMed] [Google Scholar]
- GEE J.M., DUPONT M.S., DAY A.J., PLUMB G.W., WILLIAMSON G., JOHNSON I.T. Intestinal transport of quercetin glycosides in rats involves both deglycosylation and interaction with the hexose transport pathway. J. Nutr. 2000;130:2765–2771. doi: 10.1093/jn/130.11.2765. [DOI] [PubMed] [Google Scholar]
- GEE J.M., DUPONT M.S., RHODES M.J., JOHNSON I.T. Quercetin glucosides interact with the intestinal glucose transport pathway. Free Radic. Biol. Med. 1998;25:19–25. doi: 10.1016/s0891-5849(98)00020-3. [DOI] [PubMed] [Google Scholar]
- GOLDBERG D.M., JOSEPH Y., SOLEAS G.J. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin. Biochem. 2003;36:79–87. doi: 10.1016/s0009-9120(02)00397-1. [DOI] [PubMed] [Google Scholar]
- HAKKINEN S., AURIOLA S. High-performance liquid chromatography with electrospray ionization mass spectrometry and diode array ultraviolet detection in the identification of flavonol aglycones and glycosides in berries. J. Chromatogr. A. 1998;829:91–100. doi: 10.1016/s0021-9673(98)00756-0. [DOI] [PubMed] [Google Scholar]
- HERRERA M.D., ZARZUELO A., JIMENEZ J., MARHUENDA E., DUARTE J. Effects of flavonoids on rat aortic smooth muscle contractility: structure–activity relationships. Gen. Pharmacol. 1996;27:273–277. doi: 10.1016/0306-3623(95)02010-1. [DOI] [PubMed] [Google Scholar]
- JONES D.J., LAMB J.H., VERSCHOYLE R.D., HOWELLS L.M., BUTTERWORTH M., LIM C.K., FERRY D., FARMER P.B., GESCHER A.J. Characterisation of metabolites of the putative cancer chemopreventive agent quercetin and their effect on cyclo-oxygenase activity. Br. J. Cancer. 2004;91:1213–1219. doi: 10.1038/sj.bjc.6602091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALDAS M.I., WALLE U.K., VAN DER WOUDE H., MCMILLAN J.M., WALLE T. Covalent binding of the flavonoid quercetin to human serum albumin. J. Agric. Food. Chem. 2005;53:4194–4197. doi: 10.1021/jf050061m. [DOI] [PubMed] [Google Scholar]
- KANNER J., LAPIDOT T. The stomach as a bioreactor: dietary lipid peroxidation in the gastric fluid and the effects of plant-derived antioxidants. Free Radic. Biol. Med. 2001;31:1388–1395. doi: 10.1016/s0891-5849(01)00718-3. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI Y., SUZUKI M., SATSU H., ARAI S., HARA Y., SUZUKI K., MIYAMOTO Y., SHIMIZU M. Green tea polyphenols inhibit the sodium-dependent glucose transporter of intestinal epithelial cells by a competitive mechanism. J. Agric. Food Chem. 2000;48:5618–5623. doi: 10.1021/jf0006832. [DOI] [PubMed] [Google Scholar]
- KOGA T., MEYDANI M. Effect of plasma metabolites of (+)-catechin and quercetin on monocyte adhesion to human aortic endothelial cells. Am. J. Clin. Nutr. 2001;73:941–948. doi: 10.1093/ajcn/73.5.941. [DOI] [PubMed] [Google Scholar]
- LEMOS C., AZEVEDO I., MARTEL F. Effect of red wine on the intestinal absorption of thiamine and folate in the rat: comparison with the effect of ethanol alone. Alcohol Clin. Exp. Res. 2005;29:664–671. doi: 10.1097/01.alc.0000159114.86360.b5. [DOI] [PubMed] [Google Scholar]
- LEVITT M.D., LI R., DEMASTER E.G., ELSON M., FURNE J., LEVITT D.G. Use of measurements of ethanol absorption from stomach and intestine to assess human ethanol metabolism. Am. J. Physiol. 1997;273:G951–G957. doi: 10.1152/ajpgi.1997.273.4.G951. [DOI] [PubMed] [Google Scholar]
- MA T.Y., NGUYEN D., BUI V., NGUYEN H., HOA N. Ethanol modulation of intestinal epithelial tight junction barrier. Am. J. Physiol. 1999;276:G965–G974. doi: 10.1152/ajpgi.1999.276.4.G965. [DOI] [PubMed] [Google Scholar]
- MAGNUSSEN M.P. The effect of ethanol on the gastrointestinal absorption of drugs in the rat. Acta Pharmacol. Toxicol. 1968;26:130–144. doi: 10.1111/j.1600-0773.1968.tb00433.x. [DOI] [PubMed] [Google Scholar]
- MUKAMAL K.J., ASCHERIO A., MITTLEMAN M.A., CONIGRAVE K.M., CAMARGO C.A., KAWACHI I., STAMPFER M.J., WILLETT W.C., RIMM E.B. Alcohol and risk for ischemic stroke in men: the role of drinking patterns and usual beverage. Ann. Intern. Med. 2005;142:11–19. doi: 10.7326/0003-4819-142-1-200501040-00007. [DOI] [PubMed] [Google Scholar]
- MUROTA K., TERAO J. Antioxidative flavonoid quercetin: implication of its intestinal absorption and metabolism. Arch. Biochem. Biophys. 2003;417:12–17. doi: 10.1016/s0003-9861(03)00284-4. [DOI] [PubMed] [Google Scholar]
- PELLEGRINI N., VISIOLI F., BURATTI S., BRIGHENTI F. Direct analysis of total antioxidant activity of olive oil and studies on the influence of heating. J. Agric. Food Chem. 2001;49:2532–2538. doi: 10.1021/jf001418j. [DOI] [PubMed] [Google Scholar]
- RUF J.C., BERGER J.L., RENAUD S. Platelet rebound effect of alcohol withdrawal and wine drinking in rats. Relation to tannins and lipid peroxidation. Arterioscler. Thromb. Vasc. Biol. 1995;15:140–144. doi: 10.1161/01.atv.15.1.140. [DOI] [PubMed] [Google Scholar]
- SAPONARA S., SGARAGLI G., FUSI F. Quercetin as a novel activator of L-type Ca(2+) channels in rat tail artery smooth muscle cells. Br. J. Pharmacol. 2002;135:1819–1827. doi: 10.1038/sj.bjp.0704631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGLETON V.L., ROSSI J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- SOLEAS G.J., YAN J., GOLDBERG D.M. Ultrasensitive assay for three polyphenols (catechin, quercetin and resveratrol) and their conjugates in biological fluids utilizing gas chroma-tography with mass selective detection. J. Chromatogr. B. 2001;757:161–172. doi: 10.1016/s0378-4347(01)00142-6. [DOI] [PubMed] [Google Scholar]
- YEH C.T., YEN G.C. Effects of phenolic acids on human phenolsulfotransferases in relation to their antioxidant activity. J. Agric. Food Chem. 2003;51:1474–1479. doi: 10.1021/jf0208132. [DOI] [PubMed] [Google Scholar]