Abstract
Neutrophil infiltration, proinflammatory cytokines, eicosanoid generation and oxidative stress have been implicated in colitis. Resveratrol is a polyphenolic compound found in grapes and wine, with multiple pharmacological actions, including anti-inflammatory, antioxidant, antitumour and immunomodulatory activities. In a previous report, we documented that resveratrol decreases the degree of inflammation associated with acute experimental colonic inflammation, but its effects on chronic experimental colitis remain undetermined.
The aim of this research was to investigate the effects of resveratrol on the chronic colonic injury caused by intracolonic instillation of trinitrobenzenesulphonic acid (TNBS) in rats. The inflammatory response was assessed by histology and myeloperoxidase activity. Tumour necrosis factor alpha (TNF-α) production, histological and histochemical analysis of the lesions were also carried out. We determined the production of prostaglandin (PG) E2 and D2 in colon mucosa, as well as cyclooxygenase (COX)-1 and -2 and nuclear transcription factor NF-kappa B (NF-κB) p65 protein expression. Finally, since resveratrol has been found to modulate apoptosis, we intended to elucidate its effects on colonic mucosa under chronic inflammatory conditions.
Resveratrol (10 mg kg−1 day−1) significantly attenuated the damage score and corrected the disturbances in morphology associated to injury. In addition, the degree of neutrophil infiltration and the levels of TNF-α were significantly ameliorated. Resveratrol did not modify PGD2 levels but returned the decreased PGE2 values to basal levels and also reduced COX-2 and the NF-κB p65 protein expression. Furthermore, treatment of rats with resveratrol caused a significant increase of TNBS-induced apoptosis in colonic cells.
In conclusion, resveratrol reduces the damage in chronic experimentally induced colitis, alleviates the oxidative events, returns PGE2 production to basal levels and stimulates apoptosis in colonic cells.
Keywords: Resveratrol, trinitrobenzenesulphonic acid (TNBS), neutrophils, cyclooxygenase (COX), prostaglandin (PG), nuclear transcription factor NF-kappa B (NF-κB), apoptosis
Introduction
Ulcerative colitis and Crohn's disease are the two major forms of inflammatory bowel disease (IBD). The pathogenesis of IBD is a multifactorial process. One of the earliest factors involved includes the breach of the intestinal epithelial barrier by unknown mechanisms, focus of current investigation and controversy (Schmidt & Stallmach, 2005; Thompson-Chagoyan et al., 2005). In addition, the development of an abnormal immune and inflammatory response occurs, which is mediated predominantly by activated neutrophils, monocytes and macrophages and characterised by an enhanced formation of reactive oxygen and nitrogen species. Various proinflammatory cytokines such as interleukin (IL)-1, IL-6 and tumour necrosis factor alpha (TNF-α), chemokines and adhesion molecules are known to contribute to the pathogenesis of IBD (Sandborn & Yednock, 2003; Kurtovic & Segal, 2004). In particular, TNF-α plays a crucial activating role among the cytokines that modulate endothelial functions; in fact, TNF-α regulates the expression of some adhesion molecules and cytokines on endothelial cells and also increases vascular permeability (Sasaki et al., 2004).
Inhibition of nuclear factor-kappa B (NF-κB) activation has been suggested as an anti-inflammatory treatment strategy in IBD. The nuclear transcription factor NF-κB is a key regulator of the inducible expression of many genes involved in immune and inflammatory responses in the gut. Stimuli such as oxidative stress, cytokines (IL-1, IL-6, TNF-α), bacteria and viruses can release NF-κB to allow translocation to the nucleus (Dijkstra et al., 2002).
The polyphenolic phytoalexin resveratrol (3,5,4′-trihydroxy-trans-stilbene) is a naturally occurring stilbene found in various foods, including mulberries, peanuts, grapes and the red wines. Previous reports have demonstrated that resveratrol regulates many biological activities, mainly concentrating on tumour, oxidation and inflammation regulation (Amorati et al., 2004; Baolin et al., 2004; Bode & Dong, 2004; Alarcón de la Lastra & Villegas, 2005). It is also reported to be a neuroprotective (Han et al., 2004) and cardioprotective agent (Hung et al., 2000). Indeed, resveratrol has been shown to modulate lipid oxidation and lipoprotein metabolism (Belguendouz et al., 1998). The anti-inflammatory mechanism of resveratrol is not understood completely, but a reduction of cyclooxygenases COX-1 and COX-2 expression and activity is also apparent (Martínez & Moreno, 2000; Martín et al., 2004; Szewczuk & Penning, 2004). The role of prostanoids in the intestinal inflammatory process is still controversial. We have recently reported that the increased PGE2 production during acute colitis is dependent upon the activity of COX-2 (Martín et al., 2003; 2004). On the other hand, previous reports have suggested that decrease of local PGs is correlated with colonic mucosal inflammation (Villegas et al., 2003a, 2003b). In fact, experimental colitis can be attenuated by pretreatment with exogenous PGs (Fedorak et al., 1990; Tessner et al., 1998). Moreover, recent studies have shown that inhibition of COX-2 activity during the healing process is detrimental, as it has been proven that this isoenzyme also exerts anti-inflammatory effects (Gilroy et al., 1999; Ajuebor et al., 2000). Thus, both COX-1 and COX-2 are necessary in order to yield an amelioration of the damage (Berenguer et al., 2002).
In a previous study, we have demonstrated the efficacy of this compound in early colonic inflammation (Martín et al., 2004), but its effects on chronic experimental colitis remain undetermined. Thus, here we investigated the effects of chronic treatment with resveratrol on the colon injury caused by intracolonic administration of TNBS in rats. We have studied the inflammatory response by histology and myeloperoxidase (MPO) activity, as an index of quantitative inflammation and leukocyte infiltration in the mucosa. TNF-α production, histological and histochemical analysis of the lesions were also carried out. In order to gain a better insight into the mechanism(s) of action, the observed protective effects of resveratrol, the expression of the nuclear transcription factor NF-κB p65, PGE2 and PGD2 generation, and the expression of COX-1 and -2 by Western blotting and immunohistochemistry have been investigated. Finally, since resveratrol has been found to modulate apoptosis in acute colitis (Martín et al., 2004), our aim was to study its effects in colonic mucosa under chronic inflammatory conditions.
Methods
Experimental animals
Male Wistar rats supplied by Animal Services, Faculty of Medicine, University of Seville, Spain, and weighing 180–220 g, were placed individually in cages with wire-net floors in a controlled room (temperature 24–25°C, humidity 70–75%, lighting regimen of 12L/12D) and were fed a normal laboratory diet (Panlab, Barcelona, Spain). Rats were deprived of food for 24 h prior to the induction of colitis, but were allowed free access to tap water throughout. They were randomly assigned to groups of 8–12 animals. Experiments followed a protocol approved by the local animal Ethics Committee and the Local Government. All experiments were in accordance with the recommendations of the European Union regarding animal experimentation (Directive of the European Counsel 86/609/EC).
Induction of colitis
Colitis was induced according to the procedure described by Morris et al. (1989). Briefly, rats were slightly anaesthetised with ether following a 24 h fast, and then a medical-grade polyurethane canal for enteral feeding (external diameter 2 mm) was inserted into the anus and the tip was advanced to 8 cm proximal to the anus verge. A volume of 30 mg of TNBS (Sigma-Aldrich Company Ltd, Madrid, Spain) dissolved in a volume of 0.25 ml of ethanol 50% (v v−1) was instilled into the anus, to induce chronic colitis. Following the instillation of the hapten, the animals were maintained in a head-down position for a few minutes to prevent leakage of the intracolonic instillate. Different control groups were separated for comparison with TNBS/ethanol instillation: rats in the sham group received an enema of physiological saline instead of the TNBS solution, and the ethanol group received 0.25 ml of 50% ethanol. Resveratrol (10 mg kg−1; Sigma-Aldrich Company Ltd, Spain) was suspended in 0.9% saline solution and administered by gavage (p.o.) 24 h after TNBS instillation and daily during the 2 weeks before killing of colitis. Control groups received vehicle in a comparable volume (10 ml per animal). The dose of resveratrol (10 mg kg−1) was selected taking into account previous results (Martín et al., 2004). The animals were killed, using an overdose of anesthetic, 14 days after induction of colitis. The rats were checked daily for behaviour, body weight, and stool consistency.
Assessment of colitis
The severity of colitis was evaluated by an independent observer who was blinded to the treatment. For each animal, the distal 10 cm portion of the colon was removed and cut longitudinally, slightly cleaned in physiological saline to remove faecal residues and weighed. Macroscopic inflammation scores were assigned based on clinical features of the colon (score 0–10): 0 (no damage), 1 (focal hyperaemia), 2 (ulceration without hyperaemia or bowel wall thickening), 3 (ulceration with inflammation at 1 site), 4 (=or >2 sites of ulceration and inflammation), 5 (major sites of inflammation >1 cm along the organ), 6–10 (major sites of inflammation >2 cm along the organ). The presence of adhesions between colon and small bowel and other organs (score 0–2), and/or stool consistency (score 0–1) were evaluated according to the criteria of Bobin-Dubigeon et al. (2001). Pieces of inflamed colon were collected and frozen in liquid nitrogen for measurement of biochemical parameters.
Histological studies
For examination with the light microscope, we used tissue samples from the distal colon of each animal fixed in 4% buffered paraformaldehyde, dehydrated in grade ethanol and embedded in paraffin. Thereafter, sections of tissue were cut at 5 μm on a rotary microtome (Leica Ultracut), mounted on clean glass slides and dried overnight at 37°C. Sections were cleared, hydrated and stained with haematoxylin and eosin, Giemsa and Alcian blue for histological evaluation of colonic damage, cell infiltration and mucus content, respectively, according to standard protocols, and the slides were coded to prevent observer bias during evaluation. All tissue sections were examined in an Olympus BH-2 microscope for characterization of histopathological changes. Photographs taken from colon samples were digitised using Kodak D290 Zoom camera Eastman Kodak Co., U.S.A. and Motic® Images 2000 release 1.1 (MicroOptic Industrial Group Co., Ltd; B1 Series System Microscopes). Analysis of the figures was carried out by Adobe®Photoshop® Version 5.0 (Adobe Systems) image analysis program. For measurement of the number of mucosal mast cells, the sections were stained with 0.5% toluidine blue (pH 0.5) (Wingren & Enerbäck, 1983). Light microphotographs of sections stained were taken using an Olympus BH-2 microscope (Olympus Optical Co., Ltd). To count mast cells, 10 high-power fields (× 20) were selected and the number per 10 fields was calculated.
Immunohistochemical study
Colonic tissues were fixed in 4% buffered paraformaldehyde, dehydrated through graded concentrations of ethanol, embedded in paraffin, and sectioned. Sections (5 μm thick) were mounted on slides, cleared and hydrated. All of them were treated with a buffered blocking solution (3% bovine serum albumin in phosphate-buffered saline (PBS)) for 15 min. Then, sections (except negative control) were coincubated with primary antibodies for COX-1 and COX-2 (goat polyclonal, M-19 (sc-1747) and M-20 (sc-1754), of Santa Cruz Biotechnology, Inc. (Barcelona, Spain) at a dilution of 1 : 400 at room temperature for 1 and 24 h, respectively. Sections were washed with PBS and coincubated with secondary antibody (anti-sheep IgG, peroxidase conjugated, Sigma, Spain) (1 : 500 in PBS, v v−1), at room temperature for 1 h. Thereafter, sections were washed as before and with Tris-HCl, 0.05 M, pH 7.66, and then coincubated with a 3,3′-diaminobenzidine solution in the dark, at room temperature for 10 min. Sections were washed with Tris-HCl, stained with haematoxylin according to standard protocols, mounted with glycerin and observed in an Olympus BH-2 microscope.
Assessment of leukocyte involvement
MPO activity was assessed as a marker of neutrophil infiltration according to the methods of Grisham et al. (1990). In all animals one sample from the distal colon was obtained. Samples were excised from each animal and rapidly rinsed with ice-cold saline, blotted dry and frozen at −70°C. The tissue was thawed, weighed and homogenised in 10 volumes 50 mM PBS, pH=7.4. The homogenate was centrifuged at 20,000 × g, 20 min, 4°C. The pellet was again homogenized in 10 volumes 50 mM PBS, pH=6.0, containing 0.5% hexadecyl-trimethylammonium bromide (HETAB) and 10 mM ethylenediamine tetraacetic acid (EDTA). This homogenate was subjected to one cycle of freezing/thawing and a brief period of sonication. A sample of homogenate (0.5 μl) was added to a 0.5 ml reaction volume containing 80 mM PBS, pH 5.4, 0.5% HETAB and 1.6 mM 3,3′,5,5′-tetramethylbenzidine (TMB). The mixture was incubated at 37°C for 5 min and the reaction started by the addition of 0.3 mM H2O2.
Each tube containing the complete reaction mixture was incubated for exactly 3 min at 37°C. The reaction was terminated by the sequential addition of catalase (20 μg ml−1) and 2 ml 0.2 M sodium acetate, pH=3.0. The changes in absorbance at 655 nm were measured with a spectrophotometer. MPO activity (1 U) was defined as the amount of enzyme present that produced a change in absorbance of 1.0 U min−1 at 37°C in the final reaction volume containing the acetate. Results were quantified as U × 103 mg−1 tissue.
Production of PGE2
PGE2 was determined in colon tissue samples obtained from each group following the protocol established previously (Martín et al., 2003; 2004). Briefly, colonic mucosa was excised and rapidly rinsed with ice-cold saline. The tissue was weighed and homogenised in 6 ml TEAP buffer (pH 3.24), which contained a COX inhibitor, lysine acetyl salicylate (Sigma Aldrich-Company Ltd, Spain). The homogenate was centrifuged (1500 × g, 10 min, 4°C) and the supernatant was removed and passed through a reverse-phase octadecylsilica C18 Sep Pak cartridge, which was washed with 10 ml distilled water, 10 ml 15% (v v−1) ethanol, 10 ml hexane and 10 ml ethylacetate, and the eluate collected. Each fraction was evaporated with ethylacetate, and the dry residue redissolved in ethanol. PGE2 was determined by a competitive enzyme immunoassay kit (Assay Designs, Inc.). PGE2 levels were quantified as PGE2 mg−1 tissue.
Production of PGD2
PGD2 was determined in colon tissue samples obtained from each group following the protocol established previously (Martín et al., 2003; 2004). Briefly, colonic mucosa was excised and rapidly rinsed with ice-cold saline. The tissue was weighed and homogenised in TEAP buffer (pH 3.24) which contained a COX inhibitor, lysine acetyl salicylate (Sigma-Aldrich Company Ltd, Spain). The homogenate was centrifuged (1500 × g, 10 min, 4°C) and an aliquot of supernatant had to be methoximated due to its chemical instability and rapid degradation. Later, the supernatant was removed and passed through a reverse-phase octadecylsilica C18 Sep Pak cartridge, which was washed with 10 ml distilled water, 10 ml 15% ethanol, 10 ml hexane and 10 ml ethylacetate, and the eluate collected. Each ethylacetate fraction was evaporated, and the dry residue re-dissolved in buffer. PGD2 was determined by a competitive enzyme immunoassay kit (Cayman Chemical, Barcelona, Spain). Results are expressed as PGD2 mg−1 tissue.
TNF-α production
Distal colon samples were weighed and homogenised, after thawing, in 100 mg sample per 0.9 ml PBS solution (pH 7.2) at 4°C. They were centrifuged at 6000 × g for 20 min. Mucosal TNF-α level was assayed by using a commercially available TNF-α enzyme-linked immunosorbent assay (ELISA) kit (Genzyme Diagnostics, Cambridge, U.K.). The TNF-α values were expressed as pg mg−1 tissue.
Isolation of cytoplasmic and nuclear proteins and Western blot assay
Nuclear proteins were isolated by the method of Helenius et al. (1996). Frozen colonic tissues were weighed and homogenised in ice-cold hypotonic buffer (1.5 mM MgCl2, 10 mM KCl, 0.2 mM phenylmethylsulphonyl fluoride (PMSF), 1.0 mM dithiothreitol (DTT) and 10 mM HEPES, pH 7.9). Homogenates were incubated for 10 min on ice and centrifuged (25,000 × g, 15 min, 4°C). Cytoplasmic proteins were collected from the supernatants and nuclear proteins from the pellets. These were washed once and centrifuged at 10,000 × g, 15 min, 4°C), after which they were suspended in ice-cold low-salt buffer (25% v v−1 glycerol, 1.5 mM MgCl2, 0.2 mM EDTA, 0.2 mM PMSF, 1.0 mM DTT, KCl, HEPES, pH 7.9). Nuclear proteins were released by adding a high-salt buffer (25% glycerol, 1.5 mM MgCl2, 0.2 mM EDTA, 0.2 mM PMSF, 1.0 mM DTT, 1.2 M KCl, 20 mM HEPES, pH 7.9) drop by drop to a final concentration of 0.4 M KCl. Samples were incubated on ice for 30 min, with smooth shaking. Soluble nuclear proteins were recovered by centrifugation (25,000 × g, 30 min, 4°C) and proteins were stored at −80°C.
Protein concentration of the homogenate was determined following Bradford's colorimetric method. Aliquots of supernatants containing equal amounts of protein (30 μg) were separated on 10% acrilamide gel by sodium dodecyl sulphate–polyacryamide gel electrophoresis. In the next step, the proteins were electrophoretically transferred onto a nitrocellulose membrane and incubated with specific primary antibodies (Santa Cruz Biotechnology, CA, U.S.A.) for COX-1 (M-20) at a dilution of 1 : 2000, COX-2 (M-19) at a dilution of 1 : 400 and NF-κB p65, at a dilution of 1 : 200, respectively. Each filter was washed three times for 15 min and incubated with the secondary horseradish peroxidase-linked anti-goat (for COX-1 and COX-2) or anti-rabbit immunoglobulin G (for NF-κB p65) antibodies (sc-2020, Santa Cruz Biotechnology, Inc.). To prove equal loading, the blots were analysed for β-actin expression using an anti-β-actin antibody (Santa Cruz Biotechnology, CA, U.S.A.). Immunodetection was performed using an enhanced chemiluminescence light-detecting kit (Amersham, Arlinghton Heights, IL, U.S.A.). Densitometric data were studied following normalisation to the control (house-keeping gene). The signals were analysed and quantified by a Scientific Imaging Systems (KODAK 1D Image Analysis Software).
Apoptosis
Cytoplasmic DNA fragments, which are indicators of apoptosis, were measured with a DNA cell death detection ELISA PLUS KIT (Roche Diagnostics) according to the manufacturer's instructions. Results were expressed as absorbance × 103 mg−1 tissue.
Statistical analysis
All values in the figures and text are expressed as arithmetic means±standard error (s.e.m.) of the mean. Data were evaluated with Graph Pad Prism® Version 2.01 software. The statistical significance of any difference in each parameter among the groups was evaluated by one-way analysis of variance (ANOVA), using Tukey–Kramer multiple comparisons test as post hoc test. P-values of <0.05 were considered statistically significant. In the experiment involving histology or immunohistochemistry, the figures shown are representative of at least six experiments performed on different days.
Results
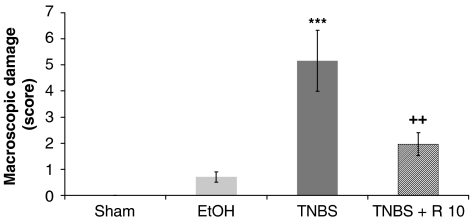
At 14 days after intracolonic administration of TNBS, the control animals underwent severe anorexia with a marked body weight loss. Colitis gave rise to diarrhoea in the majority of animals. Macroscopic inspection of the cecum, colon and rectum showed a flaccid appearance and evidence of bowel wall thickening, inflammation and ulcers. Lesions in the distal colon were quantified using a macroscopic damage score (mean: 5.1±1.1) (Figure 1). A significant increase of weight/length of the rat colon, an indicator of inflammation, and presence of adhesions to adjacent organs were frequently observed in TNBS-treated rats. However, no significant increase in the weight/length of the rat colon was observed in TNBS rats, which had been treated with resveratrol 10 mg kg−1 (Table 1). In addition, the polyphenolic compound significantly attenuated the extent and severity of the colonic injury (Figure 1). In fact, resveratrol was able to reduce the macroscopic damage score down to 2.0±0.4 (P<0.01).
Figure 1.
Effects of chronic administration of resveratrol on the colonic damage score. Colonic macroscopic damage resulting from trinitrobenzene sulphonic acid (30 mg per animal) instilled into rat colon was scored as indicated in Methods. Scores were quantified in the absence of treatment, but with daily administration of the vehicle saline solution (sham group, trinitrobenzene sulphonic acid group and ethanol group), or in the presence of resveratrol (R: 10 mg kg−1 day−1). Data are expressed as the mean±s.e.m. of 10–11 animals in each group. ***P<0.001 vs sham. ++P<0.01 vs TNBS.
Table 1.
Parameters quantified after administration of resveratrol (R: 10 mg kg−1) in rats with chronic colitis induced by TNBS intracolonic instillation (30 mg per animal)
| Group | n | Body weight changes (g) | Adhesions (score 0–2) | Stool consistency (score 0–1) | Colon weight/colon length (mg cm−1) |
|---|---|---|---|---|---|
| Sham | 10 | 88.3±16.4 | 0 | 0 | 12.1±0.8 |
| EtOH | 8 | 81.7±16.4 | 0 | 0 | 15.1±1.9 |
| TNBS | 10 | 76.0±20.7 | 1.8±0.2a,b | 0.6±0.2 | 30.4±8.4a |
| TNBS+R 10 | 11 | 78.6±13.6 | 1.6±0.1a,b | 0.3±0.2 | 22.3±2.0 |
n: number of animals per group
Colonic parameters were quantified in the sham group (n=10), which received saline instillation. TNBS group (n=8) received trinitrobenzene sulphonic acid intracolonically in a vehicle of 50% ethanol; ethanol group (n=8) received 50% ethanol intracolonic injection. Data are expressed as mean±s.e.m.
P<0.05 significantly different from sham.
P<0.05 significantly different from EtOH.
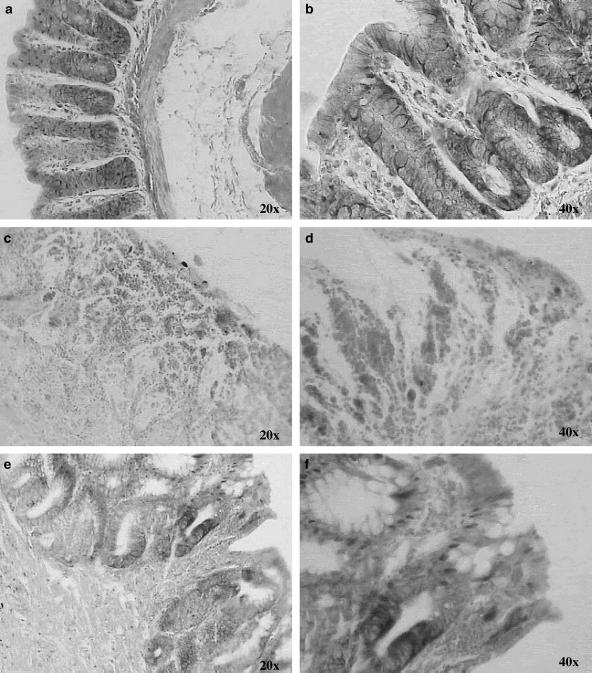
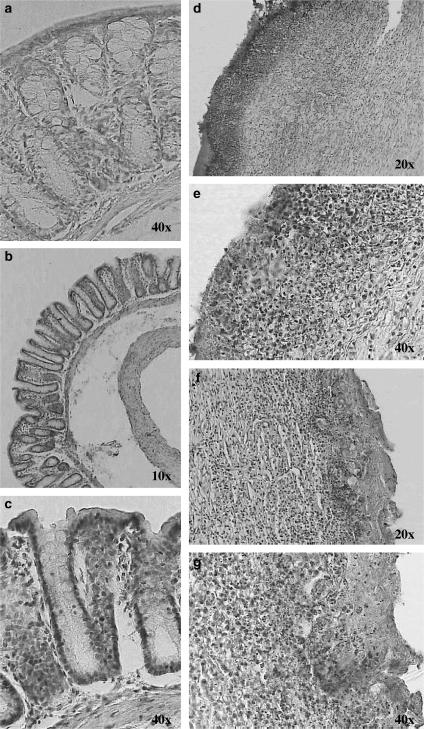
On histological examination of the colon from sham-treated rats, the histological features of the colon were typical of a normal structure (Figures 2a, b and 3a and b). In TNBS-treated rats, the inflammation extended through the mucosa, muscularis mucosae and submucosa. The mucosa adjacent to ulcers showed extensive crypt distortion. In some sections of ulcerated areas, necrotic tissue adjacent to surface cells could be observed (Figure 2c). Extensive granulation tissue with the presence of fibroblasts, monocytes and lymphocytes and inflammatory cell infiltrate, mainly eosinophils and basophils, was apparent (Figures 3c and d). Globet cells were totally absent in the mucosa (Figure 2d).
Figure 2.
Chronic colitis model induced by TNBS: effect of resveratrol on colon injury. Histological appearance of rat colonic mucosa after haematoxylin and eosin stain (e–h) or Alcian blue stain (a, b): sham (a, b), and treated with TNBS 30 mg per animal (c, d), and resveratrol 10 mg kg−1 (e, f). Histopathological features of the colon in association with colitis. (a, b) No histological modification was present in the sham animals. (c, d) Mucosal injury was produced after TNBS administration, characterised by necrosis, oedema and higher fibrosis. (e) Treatment with resveratrol (10 mg kg−1) reduced the morphological alteration associated with TNBS administration protecting the mucosal architecture. (f) Some areas showed accumulation of mucus and cell remnants however, Alcian-blue-positive cells were less numerous, and the mucin layer of the epithelium was missing. Original magnification × 10.
Figure 3.
Rat colon segments stained with Giemsa: sham (a, b), treated with TNBS 30 mg per animal (c, d), and resveratrol 10 mg kg−1 (e, f). Infiltration of inflammatory cells was highly observed in the colonic mucosa of TNBS-treated animals. Resveratrol prevented development of inflammatory changes. Original magnifications × 20 and × 40.
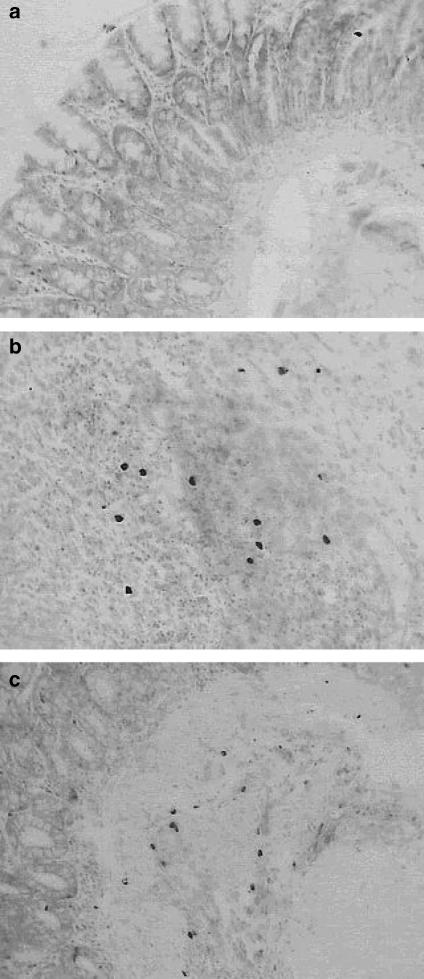
After administration of resveratrol, histologically, there was an attenuation of morphological signs of cell damage; the colonic mucosa showed ulcers in the process of healing, evolution to a more chronic inflammatory infiltrate, with mononuclear cell predominance and initiation of a repair process (Figures 2e, 3e and f). In regions with re-epithelisation of the mucosal layer, globet cells with Alcian blue-positive cells (acid glucoproteins such as sialomucins) were clearly visible (Figure 2f). Staining with toluidine blue gave a light blue background, which permitted mapping of the metachromatic mast cells with purplish blue-staining granules in their cytoplasm in relation to the other tissue components within the specimen (Figure 4). After treatment with TNBS, a marked increase in the number of mucosal mast cells was observed in the submucosa of colon with inflammation (14.7±1.8 cells per field). Treatment with resveratrol did not significantly reduce this parameter (13.0±1.0 cells per field).
Figure 4.
Rat colon segments stained with toluidine blue: sham (a), treated with TNBS 30 mg per animal (b), and treated with resveratrol 10 mg kg−1 (c). The number of mucosal mast cells was observed in the colonic submucosa of TNBS-treated animals. Resveratrol did not modify this parameter. Original magnification × 20.
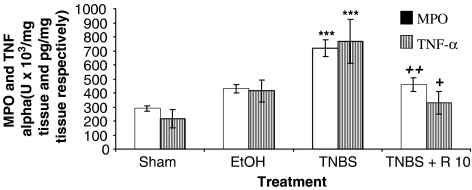
As shown in Figure 5, the colitis caused by TNBS was also characterised by a marked increase in MPO activity, an indicator of the infiltration of the colon with polymorphonuclear leukocytes. This result was consistent with the histological findings. Treatment of TNBS-treated rats with resveratrol significantly (P<0.01) reduced the degree of polymorphonuclear neutrophil infiltration. Colonic injury by TNBS administration was also characterised by an increase of the proinflammatory cytokine TNF-α. In contrast, the levels of this cytokine were significantly lower (P<0.05) in rats treated with resveratrol.
Figure 5.
MPO activity (U × 103 mg−1 tissue) and TNF-α levels (pg mg−1 tissue) after resveratrol (R: 10 mg kg−1) in rats with chronic colitis produced by TNBS intracolonic instillation (30 mg per animal). Colonic mucosal MPO activity and TNF-α levels were quantified in the absence of treatment, but with daily administration of the vehicle saline solution (sham group, trinitrobenzene sulphonic acid group and ethanol group), or in the presence of resveratrol (10 mg kg−1 day−1). Data are expressed as the mean±s.e.m. of 8–10 animals in each group. ***P<0.001 vs sham. +P<0.05 and ++P<0.01 vs TNBS. The MPO activity and TNF-α levels of colonic mucosa were quantified as described in Methods.
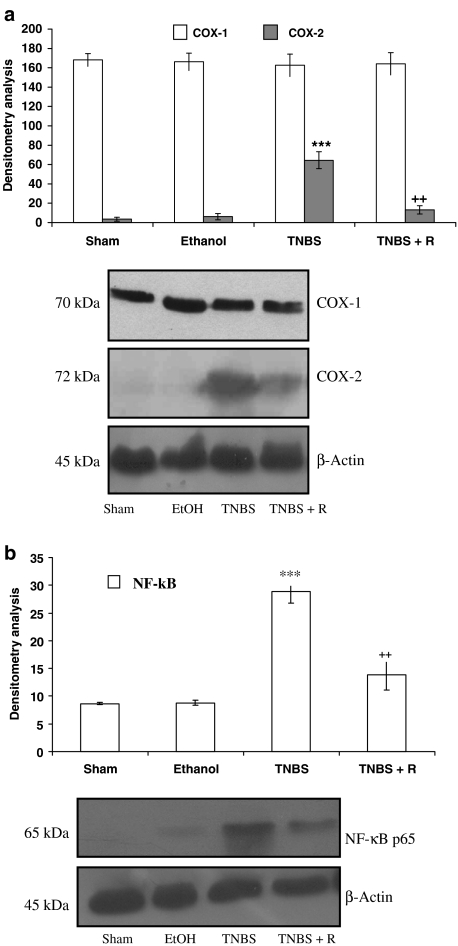
The levels of expression of NF-κB p65 and COX were measured by Western blotting of nuclear and cytosolic extracts, respectively, from colonic mucosa (Figure 6). NF-κB p65 protein was not detected in nuclei of normal colon mucosa, whereas a high expression of nuclear factor appeared in colon mucosa from control TNBS-treated rats. Nonetheless, upon treatment with resveratrol, the protein expression of NF-κB p65 was drastically decreased. As shown in this figure, the levels of COX-1 protein remained unchanged in all groups, indicating that COX-1 protein was constitutively expressed in the colonic tissue and was not significantly changed after TNBS-enema or in the presence of resveratrol. On the other hand, COX-2 protein was slightly increased by TNBS (P<0.001), indicating that the COX-2 protein expression could be induced at the chronic stage of colonic lesion caused by TNBS. Nevertheless, oral administration of resveratrol (10 mg kg−1) was able to diminish the upregulation of COX-2 (P<0.01) (Figure 6).
Figure 6.
(a) Expression of COXs after 14 days of TNBS administration as detected by Western blot analysis. COX-1 protein remained unchanged in all groups (sham, ethanol, TNBS and TNBS+resveratrol 10 mg kg−1 (R)); however, resveratrol induced downregulation of COX-2 in the treated groups vs TNBS control. Densitometric data were studied following normalisation to the control (house-keeping gene). The results are representative of three experiments performed on different samples and data are expressed as the mean±s.e.m. ***P<0.001 vs sham. ++P<0.01 vs TNBS. (b) Expression of nuclear transcription factor NF-κB p65 after 14 days of TNBS administration by Western blot analysis. The protein expression of NF-κB p65 was drastically decreased in the TNBS+resveratrol 10 mg kg−1 (R) group.
Our data showed that PGE2 content decreased significantly (P<0.05) in colonic mucosa of TNBS group compared with that of sham animals. Interestingly, under our experimental conditions, treatment with resveratrol significantly increased (P<0.001 vs TNBS) PGE2 generation and returned the PGs values to basal conditions (Table 2). In contrast, there was a significant (P<0.05) increase in the mucosal generation of PGD2 after TNBS administration; however, resveratrol did not modify PGD2 concentration.
Table 2.
Prostaglandin E2 (pg mg−1 tissue) and D2 (pg mg−1 tissue) after resveratrol administration (10 mg kg−1) in rats with chronic colitis produced by TNBS intracolonic instillation (30 mg per animal)
| Group | n | PGE2 (pg mg−1 tissue) | n | PGD2 (pg mg−1 tissue) |
|---|---|---|---|---|
| Sham | 10 | 826.3±78.4 | 8 | 803.6±18.6 |
| EtOH | 6 | 622.9±111.3 | 6 | 899.5±42.2 |
| TNBS | 10 | 444.8±98.38a | 8 | 1113.7±90.1a |
| TNBS+R 10 | 11 | 1090.9±145.7b | 9 | 983.0±72.9 |
n: number of animals per group.
Prostanoids synthesis in the colonic tissue was quantified in the absence of treatment, but with daily administration of the vehicle saline solution (sham group, trinitrobenzene sulphonic acid group and ethanol group), or in the presence of resveratrol (R: 10 mg kg−1 day−1). Data are expressed as the mean±s.e.m. The synthesis of prostanoids were quantified as described in Methods and are expressed as pg mg−1 tissue.
P<0.05 significantly different from sham.
P<0.001 significantly different from TNBS.
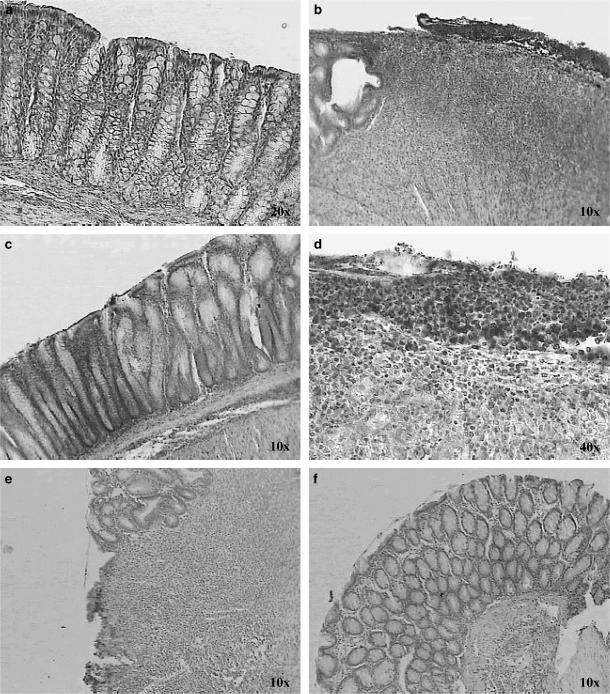
In normal colon, specific immunosignals for COX-1 were obtained in surface epithelium, and in the upper half of the crypts. Mononuclear cells of the lamina propria and the regional lymphatic nodules, as well as cells of the muscularis mucosae, showed COX-1-specific immunosignals (Figures 7b and c). In the basal part of the crypts, COX-1 expression was restricted to individual cells, which, according to morphological criteria, are endocrine cells, a specialised epithelial cell type of the lower crypt (data not shown). COX-2-specific immunolabelling was occasionally observed in colonocytes of the normal surface epithelium of matched control colon as shown in Figure 8a.
Figure 7.
Immunohistochemical localisation of COX-1 isoenzyme in sections of colon, negative control (a). In normal colon, colonocytes of the upper half of the crypts were found to be COX-1-positive (b, c). COX-1 expression in the colon of TNBS-control rats (d, e). COX-1 expression of inflamed colon treated with resveratrol 10 mg kg−1 (f, g). Original magnifications × 10, × 20 and × 40.
Figure 8.
Immunohistochemical localisation of COX-2 isoenzyme in sections of colon. COX-2 expression in normal colonic mucosa (a). COX-2 is strongly expressed in the colon of TNBS-control rats (b, d). COX-2 expression of inflamed colon treated with resveratrol 10 mg kg−1 (c, e, f).
Compared with normal colon, significant changes in the cellular distribution of COX-1 and COX-2 were observed in animals treated with TNBS; in these, cells of the surface epithelium and in the granulation tissue of mucosa were decorated by the COX-1-specific antiserum (Figures 7d and e), whereas prominent COX-2 expression was induced in cells of the surface epithelium and in cells of the inflammatory infiltrate in the area of maximum ulceration (Figures 8b and d). Compared with inflamed colon, no significant changes in the cellular localisation and the degree of positive staining for COX-1 were observed in the colon of resveratrol-treated rats (Figures 7f and g); nevertheless, oral administration of resveratrol diminished the induced upregulation of COX-2, except in apical epithelial cells of inflamed colon from resveratrol-treated rats, where there was an increase (Figures 8c, e and f).
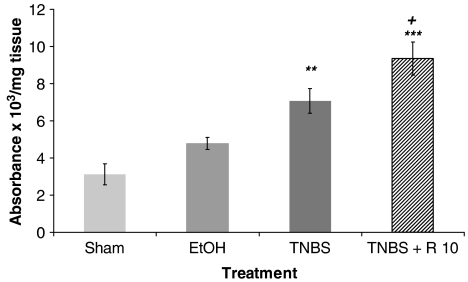
Since resveratrol has been found to modulate apoptosis, we wished to know what their effects were in colonic mucosa under chronic inflammatory conditions by an ELISA that specifically detected cytoplasmic histone-associated DNA fragments, mononucleosomes and oligonucleosomes. As shown in Figure 9, a basal level of apoptosis was observed in the colonic mucosa of sham animals. DNA fragmentation was dramatically increased in TNBS-treated rats. Furthermore, treatment of rats with resveratrol caused a significant (P<0.05) increase of TNBS-induced apoptosis.
Figure 9.
Apoptosis was observed in the colonic mucosa after acute colitis induced by TNBS (30 mg per animal). Apoptosis were quantified in the absence of treatment, but with daily administration of the vehicle saline solution (sham group, trinitrobenzene sulphonic acid group and ethanol group), or in the presence of resveratrol (R: 10 mg kg−1 day−1). Data are expressed as the mean±s.e.m. of eight to nine animals in each group. **P<0.01 and ***P<0.001 vs sham. +P<0.05 vs TNBS.
Discussion
The results of the present study indicate that daily administration of resveratrol during 14 days reduced the severity and extension of the chronic colonic damage induced by TNBS.
Our results also revealed that resveratrol increased the amount of mucus stained by Alcian blue in colon mucosa. The protective effect of mucus as an active barrier may be attributed largely to its viscous and gel-forming properties, which are derived from mucin glycoprotein constituents. Alcian-blue-positive cells seem to be associated with regenerative processes of the mucosa (Torres et al., 1999), while reduction in the amount stained has been related to a decreased resistance of the mucosa and paralleled by alterations in the normal pattern of maturation of mucin in globet cells (Einerhand et al., 2002).
Infiltration of leukocytes into the mucosa has been suggested to contribute significantly to the tissue necrosis and mucosal dysfunction associated with colitis, as they represent a major source of reactive oxygen and nitrogen species in the inflamed colonic mucosa (Liu et al., 2003; Deniz et al., 2004). Reactive oxygen species and peroxynitrite induce cellular injury and necrosis via several mechanisms, including peroxidation of membrane lipids, protein denaturation and DNA damage. Activated neutrophils produce superoxide anion, the main free radical in tissues, through NADPH oxidase, which reduces molecular oxygen to the superoxide anion radical, and through the enzyme MPO, which catalyses the formation of such potent cytotoxic oxidants as hypochlorous acid from hydrogen peroxide and chloride ions and N-chloramines.
It has been suggested that proinflammatory cytokines, such as IL-1β, interferon-γ and TNF-α, induce the production of chemoattractants for neutrophils, which regulate endothelial adhesion molecule expression. Reports from other studies (Lawrance et al., 2003; Martín et al., 2003; Kim et al., 2004) indicated that TNF-α production plays an important role in TNBS-induced chronic colitis. Our findings, as in the above-cited papers, show that MPO activity and the production of the proinflammatory cytokine TNF-α were correlated with the development of colonic inflammation. Interestingly, the ability of the polyphenolic stilbene to partially reduce the inflammatory cell infiltrate in the colon could in part explain the observed reduction in the levels of the cytokine. Similar results were obtained in a model of colitis under acute experimental conditions (Martín et al., 2004). Likewise, these results are in agreement with published in vitro data, where resveratrol has been shown to reduce the levels of these inflammatory mediators (Nawada et al., 1998; Gao et al., 2001; Estrov et al., 2003; Bi et al., 2005).
In our study, chronic inflammatory conditions were accompanied by the presence of detectable quantities of NF-κB p65 in the nuclear extracts, while the nuclear protein expression of NF-κB p65 was drastically decreased upon treatment with resveratrol. Recently, it has been suggested that the stilbene acts on NF-κB by the inhibition of the I-κB kinase, which results in the prevention of NF-κB translocation into the nucleous (Holmes-McNary & Baldwin, 2000) or through its sirtuin activity, which deacetylates NF-κB (Howitz et al., 2003; Yeung et al., 2004). The p65 subunit activation has significance in IBD because it is highly activated in the mucosal biopsy specimens of patients with ulcerative colitis and Crohn's disease (Chung et al., 2000). The p65 antisense oligonucleotide treatment also aborts chronic intestinal inflammation in a murine model of inflammation (Lawrance et al., 2003). NF-κB has been shown to activate, via transcription of genes encoding proinflammatory cytokines (TNF-α, IL-1β, IL-12 and IL-6) in different cell types, the expression of enzymes (e.g. inducible nitric oxide synthase and COX-2) (Dubuquoy et al., 2002). Thus, it is possible that one mechanism underlying the protective effects of resveratrol involves a reduction of neutrophil infiltration into the colonic mucosa, possibly via inhibition of TNF-α production and NF-κB activation, although further investigation will be required to confirm this possibility.
In the present study, we have also demonstrated that (1) a decrease in PGE2 colonic levels and an increase in PGD2 production are associated with macroscopic damage. (2) COX-2 expression is increased in colon 14 days after the induction of colitis and (3) resveratrol treatment increased PGE2 production and returned PG values to basal conditions. These results are consistent with the observation of Allgayer et al. (1989) and Tessner et al. (1998), who demonstrated that exogenously applied PGE2 attenuated experimental colitis and reduced the inflammatory response, suggesting that endogenous PGE2 is the mediator of mucosal protection. PGE2 exerts anti-inflammatory effects, including suppression of neutrophil function or prevention of mast cell degranulation, as well as suppressor T-cell induction and inhibition of the production and release of proinflammatory cytokines (Ajuebor et al., 2000). In that study, the decrease of PGE2 in the inflamed colon is consistent with a recent observation that PGE2 in caecal tissues of TNBS-treated rats increases at 1 week post-TNBS, but returns to basal values by 2 weeks post-TNBS (Zamuner et al., 2003).
Mast cells produce regulatory and immunomodulating mediators such as PG, mainly PGD2 and cytokines, and they might play an important role in the process of fibrosis and in the resolution of colonic inflammation in rats, possibly by downregulating neutrophil infiltration into the mucosa. It has further been suggested that this prostanoid is primarily derived from COX-2 (Gilroy et al., 1999). Our results showed an increase in PGD2 synthesis and COX-2 expression in relation to basal levels. The high number of mast cells found in the colon from TNBS-treated rats would explain, in part, the observed increase of PGD2 production in this group. These data are in accordance with Zamuner et al. (2003), who observed an increase in PGD2 synthesis and COX-2 expression during the period of healing of the colonic injury.
In addition, resveratrol did not significantly reduce the number of mast cells found in the submucosa and did not modify PGD2 concentration, but reduced COX-2 protein expression. These results suggest that resveratrol reduced the colonic damage by a mechanism independent of PGD2 synthesis by the COX-2 pathway. In accordance with reports in the literature, the production of this prostanoid was markedly elevated very early after induction of colitis (1–3 h), but not thereafter. The initial increase in PGD2 synthesis was paralleled by an increase in COX-2 expression, but it declined despite the continued elevation of COX-2 expression (Ajuebor et al., 2000). Resveratrol did not inhibit COX-2 activity; however, it suppressed COX-2 expression. Our data are consistent with a previous study by Kankuri et al. (2001), where the glucocorticoid dexamethasone, which did not inhibit COX-2 activity, suppressed COX-2 expression, and also reduced colonic damage and neutrophil infiltration. Indeed, 48 h after intracolonic administration, resveratrol also inhibited the expression of COX-2, without affecting its activity (Martín et al., 2004).
IBD evoke a damage-repair process accompanied by the activation of apoptotic genes. Previous studies have shown significant apoptosis in colonic epithelial cells during mild inflammation induced by TNBS, and it is suggested that expression of apoptosis markers is related to the degree of colitis (Yue et al., 2001; D'Argenio et al., 2004). In addition, deregulated apoptosis seems to be a major cause of the impaired barrier function, leading to the invasion of pathogenic microorganisms, and to an increase of leukocyte survival with exacerbation of the disease (Yue et al., 2001). These findings are in accordance with the present study, in which colonic cell death was associated with apoptosis in the colon lesion 14 days after intracolonic administration of TNBS. In addition, we have previously demonstrated that resveratrol stimulates the apoptosis during early colonic inflammation in rats (Martín et al., 2004) and now we confirm the results during chronic colonic inflammation. An increase in apoptosis linked to resveratrol has already been documented in in vitro and in vivo studies (Hayashibara et al., 2002; Bernhard et al., 2003; Boscolo et al., 2003; Cal et al., 2003; Dong, 2003; Zhou et al., 2003; Aziz et al., 2005). In these studies, it has been suggested that the p53 protein (wild type or mutant) probably is required for resveratrol-induced apoptosis. The MAP kinases, ERKs, JNKs or p38 kinases, might also be involved in resveratrol-induced activation of p53 and apoptosis. However, in certain cancer lines, resveratrol also induced apoptosis independently of p53 (Dong, 2003). The modulation of apoptosis by resveratrol in this experimental model may represent an additional protective mechanism against chronic inflammation development.
In conclusion, we have shown that resveratrol exerts protective effects in chronic experimental colitis. The anti-inflammatory effects seem to be related to impairment of neutrophil function, absence of upregulation of TNF-α and decrease of nuclear NF-κB p65 expression. Resveratrol also returned to basal values the levels of PGE2 and PGD2 and reduced the overexpression of COX-2. We also found that the polyphenolic compound caused a significant increase of TNBS-induced apoptosis. These and our previous results strongly suggest the use of resveratrol in controlling IBD.
Abbreviations
- COX
cyclooxygenase
- DTT
dithiothreitol
- EDTA
ethylenediamine tetraacetic acid
- HETAB
hexadecyl-trimethylammonium bromide
- IBD
inflammatory bowel disease
- ICAM-1
intracellular adhesion molecule-1
- IL
interleukin
- MCP-1
monocyte-chemoattractant protein-1
- MPO
myeloperoxidase
- NF-κB
nuclear factor kappa B
- PBS
phosphate-buffered saline
- PG
prostaglandin
- PMSF
phenylmethylsulphonyl fluoride
- TMB
3,3′,5,5′-tetramethylbenzidine
- TNBS
trinitrobenzenesulphonic acid
- TNF-α
tumour necrosis factor alpha
- VCAM-1
vascular cell adhesion molecule-1
References
- AJUEBOR M.N., SINGH A., WALLACE J.L. Cyclooxygenase-2-derived prostaglandin D(2) is an early anti-inflammatory signal in experimental colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G238–G244. doi: 10.1152/ajpgi.2000.279.1.G238. [DOI] [PubMed] [Google Scholar]
- ALARCÓN DE LA LASTRA C., VILLEGAS I. Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol. Nutr. Food Res. 2005;49:405–430. doi: 10.1002/mnfr.200500022. [DOI] [PubMed] [Google Scholar]
- ALLGAYER H., DESCHRYVER K., STENSON W.F. Treatment with 16,16′-dimethyl prostaglandin E2 before and after induction of colitis with trinitrobenzenesulfonic acid in rats decreases inflammation. Gastroenterology. 1989;96:1290–1300. doi: 10.1016/s0016-5085(89)80016-2. [DOI] [PubMed] [Google Scholar]
- AMORATI R., LUCARINI M., MUGNAINI V., PEDULLI G.F., ROBERTI M., PIZZIRANI D. Antioxidant activity of hydroxystilbene derivatives in homogeneous solution. J. Org. Chem. 2004;69:7101–7107. doi: 10.1021/jo0497860. [DOI] [PubMed] [Google Scholar]
- AZIZ M.H., REAGAN-SHAW S., WU J., LONGLEY B.J., AHMAD N.Chemoprevention of skin cancer by grape constituent resveratrol: relevance to human disease FASEB J. 2005191193–1195.[express article 10.1096/fj.04-3582fje. Published online April 18, 2005] [DOI] [PubMed] [Google Scholar]
- BAOLIN L., INAMI Y., TANAKA H., INAGAKI N., IINUMA M., NAGAI H. Resveratrol inhibits the release of mediators from bone marrow-derived mouse mast cells in vitro. Planta Med. 2004;70:305–309. doi: 10.1055/s-2004-818940. [DOI] [PubMed] [Google Scholar]
- BELGUENDOUZ L., FREMONT L., GOZZELINO M.T. Interaction of transresveratrol with plasma lipoproteins. Biochem. Pharmacol. 1998;55:811–816. doi: 10.1016/s0006-2952(97)00544-3. [DOI] [PubMed] [Google Scholar]
- BERENGUER B., ALARCÓN DE LA LASTRA C., MORENO F.J., MARTÍN M.J. Chronic gastric ulcer healing in rats subjected to selective and non-selective cyclooxygenase-2 inhibitors. Eur. J. Pharmacol. 2002;3:125–135. doi: 10.1016/s0014-2999(02)01494-2. [DOI] [PubMed] [Google Scholar]
- BERNHARD D., SCHWAIGER W., CRAZZOLARA R., TINHOFER I., KOFLER R., CSORDAS A. Enhanced MTT-reducing activity under growth inhibition by resveratrol in CEM-C7H2 lymphocytic leukemia cells. Cancer Lett. 2003;195:193–199. doi: 10.1016/s0304-3835(03)00157-5. [DOI] [PubMed] [Google Scholar]
- BI X.L., YANG J.Y., DONG Y.X., WANG J.M., CUI Y.H., IKESHIMA T., ZHAO Y.Q., WU C.F. Resveratrol inhibits nitric oxide and TNF-alpha production by lipopolysaccharide-activated microglia. Int. Immunopharmacol. 2005;5:185–193. doi: 10.1016/j.intimp.2004.08.008. [DOI] [PubMed] [Google Scholar]
- BOBIN-DUBIGEON C., COLLIN X., GRIMAUD N., ROBERT J.M., LE BAUT G., PETIT J.Y. Effects of tumor necrosis factor-α synthesis inhibitors on rat trinitrobenzene sulphonic acid-induced chronic colitis. Eur. J. Pharmacol. 2001;42:103–110. doi: 10.1016/s0014-2999(01)01410-8. [DOI] [PubMed] [Google Scholar]
- BODE A.M., DONG Z. Targeting signal transduction pathways by chemopreventive agents. Mutat. Res. 2004;555:33–51. doi: 10.1016/j.mrfmmm.2004.05.018. [DOI] [PubMed] [Google Scholar]
- BOSCOLO P., DEL SIGNORE A., SABBIONI E., DI GIOACCHINO M., DI GIAMPAOLO L., REALE M., CONTI P., PAGANELLI R., GIACCIO M. Effects of resveratrol on lymphocyte proliferation and cytokine release. Ann. Clin. Lab. Sci. 2003;33:226–231. [PubMed] [Google Scholar]
- CAL C., GARBAN H., JAZIREHI A., YEH C., MIZUTANI Y., BONAVIDA B. Resveratrol and cancer: chemoprevention, apoptosis, and chemo-immunosensitizing activities. Curr. Med. Chem. Anti-Cancer Agents. 2003;3:77–93. doi: 10.2174/1568011033353443. [DOI] [PubMed] [Google Scholar]
- CHUNG S.W., KANG B.Y., KIM S.H., PAK Y.K., CHO D., TRINCHIERI G., KIM T.S. Oxidized low density lipoprotein inhibits interleukin-12 production in lipopolysaccharide-activated mouse macrophages via direct interactions between peroxisome proliferator-activated receptor-γ and nuclear factor-κ B. J. Biol. Chem. 2000;275:32681–32687. doi: 10.1074/jbc.M002577200. [DOI] [PubMed] [Google Scholar]
- D'ARGENIO G., FARRACE M.G., COSENZA V., DE RITIS F., DELLA VALLE N., MANGUSO F., PIACENTINI M. Expression of apoptosis-related proteins in rat with induced colitis. Int. J. Colorect. Dis. 2004;19:451–460. doi: 10.1007/s00384-004-0585-5. [DOI] [PubMed] [Google Scholar]
- DENIZ M., CETINEL S., KURTEL H. Blood flow alterations in TNBS-induced colitis: role of endothelin receptors. Inflamm. Res. 2004;53:329–336. doi: 10.1007/s00011-004-1266-0. [DOI] [PubMed] [Google Scholar]
- DIJKSTRA G., MOSHAGE H., JANSEN PL. Blockade of NF-kappaB activation and donation of nitric oxide: new treatment options in inflammatory bowel disease. Scand. J. Gastroenterol. 2002;236 (Suppl):37–41. doi: 10.1080/003655202320621436. [DOI] [PubMed] [Google Scholar]
- DONG Z. Molecular mechanism of the chemopreventive effect of resveratrol. Mutat. Res. 2003;523–524:145–150. doi: 10.1016/s0027-5107(02)00330-5. [DOI] [PubMed] [Google Scholar]
- DUBUQUOY L., DHARANCY S., NUTTEN S., PETTERSSON S., AUWERX J., DESREUMAUX P. Role of peroxisome proliferator-activated receptor gamma and retinoid X receptor heterodimer in hepatogastroenterological diseases. Lancet. 2002;2:1410–1418. doi: 10.1016/S0140-6736(02)11395-X. [DOI] [PubMed] [Google Scholar]
- EINERHAND A.W., RENES I.B., MAKKINK M.K., VAN DER SLUIS M., BULLER H.A., DEKKER J. Role of mucins in inflammatory bowel disease: important lessons from experimental models. Eur. J. Gastroenterol. Hepatol. 2002;14:757–765. doi: 10.1097/00042737-200207000-00008. [DOI] [PubMed] [Google Scholar]
- ESTROV Z., SHISHODIA S., FADERL S., HARRIS D., VAN Q., KANTARJIAN HM., TALPAZ M., AGGARWAL B.B. Resveratrol blocks interleukin-1{beta}-induced activation of the nuclear transcription factor NF-{kappa}B, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukaemia cells. Blood. 2003;102:987–995. doi: 10.1182/blood-2002-11-3550. [DOI] [PubMed] [Google Scholar]
- FEDORAK R.N., EMPEY L.R., MACARTHUR C., JEWELL L.D. Misoprostol provides a colonic mucosal protective effect during acetic acid-induced colitis in rats. Gastroenterology. 1990;98:615–625. doi: 10.1016/0016-5085(90)90280-e. [DOI] [PubMed] [Google Scholar]
- GAO X., XU Y.X., JANAKIRAMAN N., CHAPMAN R.A., GAUTAM S.C. Immunomodulatory activity of resveratrol: suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production. Biochem. Pharmacol. 2001;62:1299–1308. doi: 10.1016/s0006-2952(01)00775-4. [DOI] [PubMed] [Google Scholar]
- GILROY D.W., COLVILLE-NASH P.R., WILLIS D., CHIVERS J., PAUL-CLARK M.J., WILLOUGHBY D.A. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- GRISHAM M.B., BENIOT J.N., GRANGER D.N. Assessment of leukocyte involvement during ischemia and reperfusion of intestine. Methods Enzymol. 1990;186:729–742. doi: 10.1016/0076-6879(90)86172-r. [DOI] [PubMed] [Google Scholar]
- HAN Y.S., ZHENG W.H., BASTIANETTO S., CHABOT J.G., QUIRION R. Neuroprotective effects of resveratrol against beta-amyloid-induced neurotoxicity in rat hippocampal neurons: involvement of protein kinase C. Br. J. Pharmacol. 2004;141:997–1005. doi: 10.1038/sj.bjp.0705688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHIBARA T., YAMADA Y., NAKAYAMA S., HARASAWA H., TSURUDA K., SUGAHARA K., MIYANISHI T., KAMIHIRA S., TOMONAGA M., MAITA T. Resveratrol induces downregulation in survivin expression and apoptosis in HTLV-1-infected cell lines: a prospective agent for adult T cell leukemia chemotherapy. Nutr. Cancer. 2002;44:193–201. doi: 10.1207/S15327914NC4402_12. [DOI] [PubMed] [Google Scholar]
- HELENIUS M., HANNINEN M., LEHTINEK S.K., SALMINEN A. Changes associate with aging and replicative senescence in the regulation of transcription factor nuclear factor-κB. Biochem. J. 1996;318:603–608. doi: 10.1042/bj3180603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMES-MCNARY M., BALDWIN A.S.JR. Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res. 2000;60:3477–3483. [PubMed] [Google Scholar]
- HOWITZ K.T., BITTERMAN K.J., COHEN H.Y., LAMMING D.W., LAVU S., WOOD J.G., ZIPKIN R.E., CHUNG P., KISIELEWSKI A., ZHANG L.L., SCHERER B., SINCLAIR D.A. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- HUNG L.M., CHEN J.K., HUANG S.S., LEE R.S., SU M.J. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc. Res. 2000;47:549–555. doi: 10.1016/s0008-6363(00)00102-4. [DOI] [PubMed] [Google Scholar]
- KANKURI E., VAALI K., KORPELA R., PAAKKARI I., VAPAATALO H., MOILANEN E. Effects of a COX-2 preferential agent nimesulide on TNBS-induced acute inflammation in the gut. Inflammation. 2001;25:301–309. doi: 10.1023/a:1012860509440. [DOI] [PubMed] [Google Scholar]
- KIM S.W., CHOI S.C., CHOI E.Y., KIM K.S., OH J.M., LEE H.J., OH H.M., KIM S., OH B.S., KIMM K.C., LEE M.H., SEO G.S., KIM T.H., OH H.C., WOO W.H., KIM Y.S., PAE H.O., PARK D.S., CHUNG H.T., JUN C.D. Catalposide, a compound isolated from catalpa ovata, attenuates induction of intestinal epithelial proinflammatory gene expression and reduces the severity of trinitrobenzene sulfonic acid-induced colitis in mice. Inflamm. Bowel Dis. 2004;10:564–572. doi: 10.1097/00054725-200409000-00010. [DOI] [PubMed] [Google Scholar]
- KURTOVIC J., SEGAL I. Recent advances in biological therapy for inflammatory bowel disease. Trop. Gastroenterol. 2004;25:9–14. [PubMed] [Google Scholar]
- LAWRANCE I.C., WU F., LEITE A.Z., WILLIS J., WEST G.A., FIOCCHI C., CHAKRAVARTI S. A murine model of chronic inflammation-induced intestinal fibrosis down-regulated by antisense NF-kappa B. Gastroenterology. 2003;125:1750–1761. doi: 10.1053/j.gastro.2003.08.027. [DOI] [PubMed] [Google Scholar]
- LIU L., MEI Q.B., ZHOU S.Y., HAN F.H., LONG Y., LIU J.Y., LI C., MENG J.R., WANG Z.P. Effects of tanguticum maxim polysaccharide on ulcerative colitis induced by TNBS in rats. Zhongguo Zhong Yao Za Zhi. 2003;28:246–249. [PubMed] [Google Scholar]
- MARTÍN A.R., VILLEGAS I., LA CASA C., ALARCÓN DE LA LASTRA C. The cyclo-oxygenase-2 inhibitor, rofecoxib, attenuates mucosal damage due to colitis induced by trinitrobenzene sulphonic acid in rats. Eur. J. Pharmacol. 2003;481:281–291. doi: 10.1016/j.ejphar.2003.09.033. [DOI] [PubMed] [Google Scholar]
- MARTÍN A.R., VILLEGAS I., LA CASA C., ALARCÓN DE LA LASTRA C. Resveratrol, a polyphenol found in grapes, suppresses oxidative damage and stimulates apoptosis during early colonic inflammation in rats. Biochem. Pharmacol. 2004;67:1399–1410. doi: 10.1016/j.bcp.2003.12.024. [DOI] [PubMed] [Google Scholar]
- MARTÍNEZ J., MORENO J.J. Effect of resveratrol, a natural polyphenolic compound, on reactive oxygen species and prostaglandin production. Biochem. Pharmacol. 2000;59:865–870. doi: 10.1016/s0006-2952(99)00380-9. [DOI] [PubMed] [Google Scholar]
- MORRIS G.P., BECK P.L., HERRIDGE M.S., DEPEW W.T., SZEWCZUK M.R., WALLACE J.L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- NAWADA N., SEKI S., INOUE M., KUROKI T. Effect of antioxidants, resveratrol, quercetin, and N-acetykcysteine, on the functions of cultural rat hepatic stellate cells and Kupffer cells. Hepatology. 1998;27:1265–1274. doi: 10.1002/hep.510270512. [DOI] [PubMed] [Google Scholar]
- SANDBORN W.J., YEDNOCK T.A. Novel approaches to treating inflammatory bowel disease: targeting alpha-4 integrin. Am. J. Gastroenterol. 2003;98:2372–2382. doi: 10.1111/j.1572-0241.2003.08703.x. [DOI] [PubMed] [Google Scholar]
- SASAKI M., ELROD J.W., JORDAN P., ITOH M., JOH T., MINAGAR A., ALEXANDER J.S. CYP450 dietary inhibitors attenuate TNF-alpha-stimulated endothelial molecule expression and leukocyte adhesion. Am. J. Physiol. Cell Physiol. 2004;286:C931–C939. doi: 10.1152/ajpcell.00351.2003. [DOI] [PubMed] [Google Scholar]
- SCHMIDT C., STALLMACH A. Etiology and pathogenesis of inflammatory bowel disease. Minerva Gastroenterol. Dietol. 2005;51:127–145. [PubMed] [Google Scholar]
- SZEWCZUK L.M., PENNING T.M. Mechanism-based inactivation of COX-1 by red wine m-hydroquinones: a structure–activity relationship study. J. Nat. Prod. 2004;67:1777–1782. doi: 10.1021/np0498410. [DOI] [PubMed] [Google Scholar]
- TESSNER T.G., COHN S.M., SCHLOEMANN S., STENSON W.F. Prostaglandins prevent decreased epithelial cell proliferation associated with dextran sodium sulfate injury in mice. Gastroenterology. 1998;115:874–882. doi: 10.1016/s0016-5085(98)70259-8. [DOI] [PubMed] [Google Scholar]
- THOMPSON-CHAGOYAN O.C., MALDONADO J., GIL A. Aetiology of inflammatory bowel disease (IBD): role of intestinal microbiota and gut-associated lymphoid tissue immune response. Clin. Nutr. 2005;24:339–352. doi: 10.1016/j.clnu.2005.02.009. [DOI] [PubMed] [Google Scholar]
- TORRES M.I., GARCÍA-MARTÍN M., FERNÁNDEZ M.I., NIETO N., GIL A., RÍOS A. Experimental colitis induced by trinitrobenzenesulphonic acid. An ultrastructural and histochemical study. Digest. Dis. Sci. 1999;44:2523–2529. doi: 10.1023/a:1026651408998. [DOI] [PubMed] [Google Scholar]
- VILLEGAS I., ALARCÓN DE LA LASTRA C., ORJALES A., LA CASA C. A new flavonoid derivative, dosmalfate, attenuates the development of dextran sulphate sodium-induced colitis in mice. Int. Immunopharmacol. 2003a;3:1731–1741. doi: 10.1016/j.intimp.2003.07.002. [DOI] [PubMed] [Google Scholar]
- VILLEGAS I., LA CASA C., ORJALES A., ALARCÓN DE LA LASTRA C. Effects of dosmalfate, a new cytoprotective agent, on acute and chronic trinitrobenzene sulphonic acid-induced colitis in rats. Eur. J. Pharmacol. 2003b;460:209–218. doi: 10.1016/s0014-2999(02)02949-7. [DOI] [PubMed] [Google Scholar]
- WINGREN U., ENERBÄCK L. Mucosal mast cells of the rat intestine: a re-evaluation of fixation and staining properties, with special reference to protein blocking and solubility of the granular glycosaminoglycan. Histochem J. 1983;15:571–582. doi: 10.1007/BF01954148. [DOI] [PubMed] [Google Scholar]
- YEUNG F., HOBERG J.E., RAMSEY C.S., KELLER M.D., JONES D.R., FRYE R.A., MAYO M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUE G., LAI P.S., YIN K., SUN F.F., NAGELE R.G., LIU X., LINASK K.K., WANG C., LIN K.T., WONG P.Y. Colon epithelial cell death in 2,4,6-trinitrobenzenesulfonic acid-induced colitis is associated with increased inducible nitric-oxide synthase expression and peroxynitrite production. J. Pharmacol. Exp. Ther. 2001;297:915–925. [PubMed] [Google Scholar]
- ZAMUNER S.R., WARRIER N., BUREO A.G., MACNAUGHTON W.K., WALLACE J.L. Cyclooxygenase 2 mediates post-inflammatory colonic secretory and barrier dysfunction. Gut. 2003;52:1714–1720. doi: 10.1136/gut.52.12.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU H.B., YAN Y., SUN Y.N., ZHU J.R. Resveratrol induces apoptosis in human esophageal carcinoma cells. World J. Gastroenterol. 2003;9:408–411. doi: 10.3748/wjg.v9.i3.408. [DOI] [PMC free article] [PubMed] [Google Scholar]