Abstract
Risk factors for cardiovascular diseases (CVD) have been proposed to accelerate the vascular endothelial dysfunction that develops during the normal ageing process. The objective of this work was to study the impact of dyslipidaemia (DL) on the dilatory efficacy of the non-NO/non-PGI2 endothelium-derived hyperpolarising factor (EDHF) through maturation and ageing.
We isolated and pressurised (80 mmHg) gracilis arterial segments from 3, 12 and 20-month-old (m/o) DL mice expressing the human apolipoprotein B-100 and wild-type (WT) C57BL/6 mice. EDHF-dependent dilatations to acetylcholine (ACh) were measured in the presence of L-NNA (100 μM, NOS inhibitor) and indomethacin (INDO; 10 μM, COX inhibitor). Data are expressed as mean±s.e.m.
EDHF-mediated maximal dilatation of arteries isolated from WT mice declined by 44% with ageing, from 86±3% at 3 months to 66±8% at 12 and 48±4% at 20 months of age (P<0.05). This decline was magnified by DL to 73%, characterised by an early increased efficacy at 3 m/o (95±2%, P<0.05) and a worsening of the dysfunction at 20 m/o (26±2%, P<0.05).
17-Octadecynoic acid (17-ODYA), a cytochrome P450/epoxygenase inhibitor, reduced by 56% (P<0.05) ACh-induced EDHF-dependent dilatation of arteries isolated from 3 m/o DL – but not WT – mice, an effect of 17-ODYA disappearing in older DL mice. 17-ODYA, however, reduced (P<0.05) ACh-induced EDHF-dependent dilatation in arteries isolated from 12 m/o WT mice by 35% and from 20 m/o WT mice by 31% (P<0.05).
Reactive oxygen species production was increased in arteries isolated from 12 m/o DL mice. The antioxidant N-acetyl-L-cystein (NAC) restored the 17-ODYA-sensitive responses in arteries isolated from 12 – but not 20 – m/o DL mice (84±3% from an Emax of 57±8%; P<0.05). NAC did not affect the dilatation of arteries isolated from WT mice.
Our data suggest that the decline in EDHF-dependent dilatation is hastened by DL despite the early expression of a 17-ODYA-sensitive pathway increasing the efficacy of the non-NO/non-PGI2 endothelium-dependent dilatation. Acute free radical production contributes to the endothelial dysfunction in the presence of DL only, by abrogating this latter pathway. This 17-ODYA-sensitive pathway, however, appears in 12 m/o WT mice and remains active at 20 m/o.
Keywords: Endothelium, gracilis artery, EDHF, hypercholesterolaemia, triglycerides, ageing, reactive oxygen species, mouse
Introduction
The endothelium-derived hyperpolarising factor (EDHF) cannot be defined as a single factor. Different candidates have been proposed as potential EDHFs and over the years, K+ ions, epoxyecosatrienoic acids (EETs) and direct electrical transfer through gap junctions have emerged as the most likely candidates (Edwards et al., 1998; Brandes et al., 2000; Dora et al., 2003). Recently, we reported that in the C57BL/6 mouse gracilis artery, EDHF-dependent dilatations depend on the activation of endothelial small and intermediate conductance calcium-dependent potassium channels (SKCa and IKCa), leading to the activation of the smooth muscle via Na+/K+-ATPase pump and, to a lesser extent, Ba2+-sensitive potassium channels (Kir) activation; neither gap junctions nor EETs contributed to acetylcholine (ACh)-induced dilatation of resistance arteries isolated from 3-month-old (m/o) C57BL/6 mice (Krummen et al., 2005).
EDHF is the major contributor to endothelium-dependent dilatations induced by agonists such as ACh and bradykinin in small arteries (Nagao et al., 1992; Véquaud & Thorin, 2001; Krummen et al., 2005). The dilatory function of the endothelium is known to decline with age (Marin, 1995; Gerhard et al., 1996; Rodriguez-Martinez et al., 1998; Csiszar et al., 2002). In most cases, this diminished endothelium-dependent dilatation has been attributed to a reduced release, formation and/or increased degradation of NO or PGI2 (van der Loo et al., 2000; Woodman et al., 2003), while it was reported that EDHF-dependent dilatation declines in some arteries (Fujii et al., 1993; Urakami-Harasawa et al., 1997) but not others (Muller-Delp et al., 2002).
Risk factors for cardiovascular diseases (CVDs) are associated with an early expression of the endothelial dysfunction. Previous studies on diabetes, hypertension, atherosclerosis and hypercholesterolaemia likewise examined possible alterations in NO and PGI2 responses (Creager et al., 1990; Kolodgie et al., 1990; Flavahan, 1992; Najibi et al., 1994; Vanhoutte & Scott-Burden, 1994; Brandes et al., 1997). In contrast, the impact of these risk factors on EDHF-mediated dilatation is not well characterised. It was reported that hypercholesterolaemia magnified the role of K+ channels in the dilatation induced by ACh (Najibi et al., 1994). In the same connexion, we recently reported that EDHF-mediated dilatation was magnified in dyslipidaemic (DL) 3 m/o mice due to a duplication of its pathways with the expression of an EET-dependent component (Krummen et al., 2005). Similar results have been reported in spontaneously hypertensive rats (SHR) where cytochrome P450 metabolites contribute to the augmented dilatation dependent of EDHF (Bussemaker et al., 2003). The knowledge on the impact of the combined expression of risk factors for CVDs and age on endothelial function is limited. Bussemaker and co-workers (2003) reported that aged SHR exhibited a selective loss of EDHF-mediated relaxation in the renal artery. The objective of the present study was therefore to examine the effects of age associated with DL on EDHF-induced dilatations of mouse gracilis arteries. Our results suggest that a clinically relevant DL leads to a selective and faster reduction in EDHF-mediated dilatation compared to the normal situation in WT despite the early expression of an EDHF alternative pathway sensitive to 17-octadecynoic acid (17-ODYA). Acute oxidative stress appears to be central to this process in DL.
Methods
Vascular preparation
The procedures and protocols were in accordance with our institutional guidelines and the Guide for the Care and Use of Laboratory Animals of Canada. Experiments were conducted on isolated gracilis arteries isolated from 3, 12 and 20 m/o DL male mice expressing the human apolipoprotein B-100 (31±1, 43±2 and 41±3 g body weight, respectively; Sanan et al., 1998; Krummen et al., 2005) and their WT C57Bl/6 control male mice (27±1, 45±1 and 53±4 g body weight, respectively) using a method described previously (Nguyen et al., 1999). Total cholesterol (mg dl−1) and triglycerides (mg dl−1) were significantly (P<0.05) elevated in DL at 3 and 12 months of age (3 m/o: 159±14 and 204±38, respectively; n=5; 12 m/o: 227±17 and 198±39, respectively; n=6) compared to WT mice (3 m/o: 107±8 and 147±24, respectively; n=6; 12 m/o: 102±6 and 92±7, respectively; n=6). At 20 months of age, cholesterol levels were similar in DL and WT mice (175±31 and 147±15 respectively; n=4–6), while triglycerides remained elevated (P<0.05) in DL (140±47; n=5) compared to WT (64±7; n=6). In a limited number of animals (n=4 per group), blood pressure was measured under anaesthesia (pentobabitone-Na, 65 mg kg−1) using a Millar catheter and revealed a similar systolic blood pressure (89±3 and 87±1 mmHg), heart rate (351±16 and 395±39 b.p.m.) and +dP/dt (4330±198 and 4550±166 mmHg s−1) in WT and DL mice, respectively.
Mice were killed by CO2 inhalation. The right or left gracilis artery was dissected and placed in ice-cold physiological salt solution (PSS) of the following composition (mM): NaCl 130, KCl 4.7, KH2PO4 1.18, MgSO4 1.17, NaHCO3 14.9, CaCl2 1.6, EDTA 0.023 and glucose 10, aerated with 12% O2/5% CO2/83% N2 (37°C, pH 7.4). All experiments were conducted on segments of 2–3 mm in length with an average internal diameter of 207±2 μm (WT) and 210±2 μm (DL) when pressurised at 80 mmHg. An equilibration time of 45 min was allowed before every experiment.
Experimental protocols
Dilatations to ACh (1 nM–30 μM) were measured in vessels precontracted with phenylephrine (PE, 1–10 μM). Following precontraction, vessels isolated from 3, 12 and 20 m/o WT mice had average diameters of 54±3, 53±1 and 52±1 μm, respectively, and 52±3, 48±1 and 47±2 μm, respectively, for those isolated from DL mice. Some pharmacological tools induced a vasoconstriction per se (see Table 2). Thus, the concentration of PE was reduced to 1 μM to reach a similar level of precontraction in all experimental conditions.
Table 2.
Efficacy (Emax) and sensibility (pD2) to ACh of the gracilis artery isolated from 3, 12 and 20 m/o WT and DL mice
| Conditions | Age | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 m/o WT | 12 m/o WT | 20 m/o WT | 3 m/o DL | 12 m/o DL | 20 m/o DL | |||||||
| Emax | pD2 | Emax | pD2 | Emax | pD2 | Emax | pD2 | Emax | pD2 | Emax | pD2 | |
| 40 mM KCL-PSS | 48±4* (6) | 7.7±0.1 | 30±5*† (6) | 7.9±0.2* | 26±2*† (5) | 5.2±0.1*†‡ | 46±4* (6) | 7.6±0.3 | 30±5† (8) | 7.8±0.1* | 24±5* (4) | 6.9±0.5 |
| INDO+L-NNA | 86±3 (7) | 7.4±0.2 | 66±8† (6) | 6.9±0.2 | 48±4†‡ (5) | 5.7±0.1†‡ | 95±2¶ (7) | 7.4±0.1 | 57±8† (8) | 6.3±0.1† | 26±2†‡¶ (5) | 6.3±0.1† |
| +17-ODYA | 89±5 (4) | 7.6±0.4 | 43±6*† (7) | 7.2±0.2 | 33±2*†‡ (3) | 5.4±0.2†‡ | 42±11*¶ (6) | 7.2±0.3 | 57±9 (6) | 5.7±0.2*† | 23±3†‡¶ (5) | 5.4±0.5†‡ |
| +Ouabain | 19±7* (4) | 6.2±0.6* | 22±5* (6) | 7.2±0.2 | 10±1*†‡ (3) | 6.3±0.3‡ | 75±10¶ (7) | 6.9±0.2 | 26±6*† (4) | 5.9±1.0 | 2±1*†‡¶ (7) | NM |
| +Apamin+Chtx | 22±4* (7) | 6.8±0.2 | 46±2*† (6) | 7.2±0.1 | 12±3*‡ (3) | NM | 25±5* (5) | 6.0±0.4* | 8±2*†‡ (4) | NM | 2±1*†‡¶ (5) | NM |
| +NAC | NT | NT | 66±10 (6) | 7.0±0.3 | 42±8 (3) | 5.4±0.1 | 88±5 (3) | 8.1±0.5 | 84±3*¶ (6) | 7.1±0.2* | 27±5‡¶ (4) | 6.4±0.3 |
Data are expressed as mean±s.e.m. Vessels were preconstricted by 40 mM KCl-PSS or by PE in the presence of INDO (10 μM) and L-NNA (100 μM) combined with apamin and charbdotoxin, 17-ODYA, ouabain or NAC.
P<0.05 compared to INDO+L-NNA.
P<0.05 compared to 3 m/o.
P<0.05 compared to 12 m/o.
P<0.05 compared to WT.
NT=not tested.
NM=not measurable.
When EDHF was studied, Nω-nitro-L-arginine (L-NNA, 100 μM) and indomethacin (INDO, 10 μM) were present in the bath chamber to prevent NO and prostanoid formation, respectively. Combined NO/PGI2-dependent dilatations to ACh were obtained in the presence of 40 mM KCl PSS and without the addition of INDO and L-NNA. Depending on the channel, enzyme or pump targeted, apamin (Apa, 1 μM), charybdotoxin (Chtx, 0.1 μM), iberiotoxin (0.1 μM), 17-ODYA, (10 μM), 14,15-epoxyeicosa-5(Z)-enoic acid (EEZE, 1 μM), barium (Ba2+, 30 μM), ouabain (1 mM), probucol (10 μM) or N-acetyl-L-cysteine (NAC, 1 μM) were added to the bath 30 min before the start of the protocol.
Free radical measurement
Isolated and pressurised gracilis arteries were incubated in the presence of 5-(and-6)-chloromethyl-2′,7′-dichlorodihdrofluorescein diacetate acetyl ester (CM-H2DCF-DA; Chaytor et al., 2003; Miura et al., 2003) added to the bath 30 min before the beginning of the experiment. Vessels were then washed three times, preconstricted with angiotensin-II (10 μM) while recording changes in fluorescence intensity. Fluorescence intensity was collected with an Ionoptix Aquire system (IonOptix, Milton, MA, U.S.A.). Fluorescence intensities at 493 nm excitation were measured at 520 nm. Measurement of fluorescence intensity was assayed in the presence of L-NNA (100 μM). Before each experiment, basal fluorescence intensity was recorded. Results represent difference between recorded and basal intensity.
Statistical analysis
In every case, n refers to the number of animals used in each protocol. Half-maximum effective concentration (EC50) of ACh was measured from each individual concentration–response curve using a logistic curve-fitting program (Microcal™Origin™ version 5.0). The pD2 value, the negative log of the EC50, was obtained. Continuous variables are expressed as mean±standard error of the mean (s.e.m.). For each protocol, basal diameter in no-flow condition was determined at the end of the 45 min-equilibration period. Myogenic tone, which is a reduction in diameter induced by an increase in luminal pressure, was measured and expressed as a percentage of the maximal diameter obtained at the end of the experiment using a Ca2+-free PSS containing 10 μM of sodium nitroprusside. ACh-induced dilatation is expressed as a percentage of the preconstriction. ANOVA were performed to compare concentration–response curves. Differences were considered to be statistically significant when the P-value was <0.05 (Scheffe's F-test).
Drugs
ACh, apamin, INDO, L-NNA, phenylephrine, NAC, charybdotoxin, ouabain, probucol and 17-ODYA were purchased from Sigma (Oakville, ON, Canada). CM-H2DCFDA was purchase from Molecular probe (Invitrogen, Burlington, ON, Canada) and was diluted daily in DMSO. Barium was purchased from Mallinckrodt. All drugs were prepared daily and diluted in water, except for INDO, EZEE, probucol and 17-ODYA, which were prepared as stock solutions and diluted in ethanol. All drugs were then directly inserted in the bath chamber and the final concentration of ethanol never exceeded 0.1%. Equimolar amounts of NaCl were replaced with KCl to prepare the 40 mM K+-PSS.
Results
Myogenic tone
The myogenic tone increased with age both in vessels isolated from WT and from DL mice (Table 1). Whereas combined NOS and COX inhibition did not affect basal tone, combined inhibition of SKCa and IKCa induced a contraction. This contractile effect of apamin and charybdotoxin was lost in old WT mice but remained persistent and greater in 12 and 20 m/o DL mice. Finally, whereas ouabain constricted at all ages in both groups, 17-ODYA increased tone significantly in vessels isolated from 20 m/o DL mice only.
Table 1.
Impact of the pharmacological agents on the myogenic tone of the gracilis artery isolated from 3, 12 and 20 m/o WT and DL mice
| Conditions | WT | DL | ||||
|---|---|---|---|---|---|---|
| 3 m/o | 12 m/o | 20 m/o | 3 m/o | 12 m/o | 20 m/o | |
| Myogenic tone (control conditions) | 14±6 (6) | 32±6† (6) | 22±7 (5) | 6±3 (6) | 27±8 (8) | 30±11† (5) |
| INDO+L-NNA | 7±1 (7) | 29±6† (6) | 19±6 (5) | 6±2 (7) | 28±8† (6) | 31±10† (5) |
| +Apamin+Chtx | 26±7* (7) | 57±6*† (6) | 36±16 (3) | 30±12* (5) | 74±10*† (4) | 77±6*† (4) |
| +17-ODYA | 23±9 (4) | 28±6 (7) | 33±16 (3) | 15±10 (6) | 45±11 (6) | 55±8† (5) |
| +Ouabain | 55±14* (4) | 55±11* (6) | 36±28 (3) | 72±9* (7) | 70±5* (6) | 63±3* (4) |
Data are expressed as mean±s.e.m. All solutions contained INDO (10 μM) and L-NNA (100 μM), except in control conditions.
P<0.05 compared to INDO+L-NNA.
P<0.05 compared to 3 m/o.
EDHF in ageing DL mouse gracilis arteries
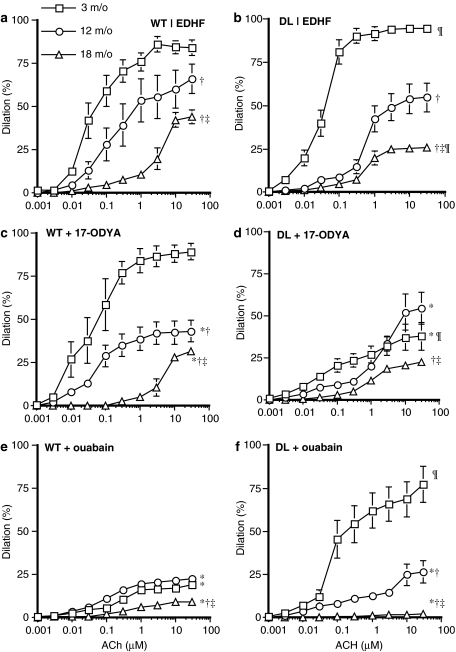
The endothelium-dependent dilatation to ACh independent of NO and PGI2 gradually decreased with increasing age (Figure 1a, Table 2). This dysfunction was also present in DL mouse gracilis arteries but with differences compared to WT (Figure 1b, Table 2). First, in vessels isolated from 3 m/o DL mice, the efficacy of ACh was greater (P<0.05) when compared to that measured in arteries isolated from WT mice. Second, the decrease in EDHF-dependent dilatation was greater (P<0.05) at 20 m/o in arteries isolated from DL compared to WT mice.
Figure 1.
ACh-induced dilatation of gracilis arteries isolated from 3, 12 and 20 m/o WT (left panels) and DL (right panels) mice. (a and b) In control conditions (n=7, 8 and 5, respectively), (c and d) in the presence of 17-ODYA (10 μM; n=6, 6 and 5, respectively) and (e and f) in the presence of ouabain (1 mM; n=7, 6 and 4, respectively). L-NNA (100 μM) and INDO (10 μM) were present in the bath. *P<0.05 compared to EDHF (a or b); †P<0.05 compared to 3 m/o; ‡P<0.05 compared to 12 m/o; ¶P<0.05 compared to WT, same age and experimental conditions.
NO and PGI2 dilatations
NO/PGI2-induced dilatations to ACh, revealed on a preconstriction induced by a 40 mM KCl-PSS, were 50% less potent than EDHF-induced dilatations (Table 2). NO/PGI2-induced dilatations were also less affected by ageing and DL than EDHF-dependent dilatations. NO/PGI2-induced maximal dilatations were reduced (P<0.05) to 30±5% at 12 months of age but were then maintained in arteries of 20 m/o mice (Table 2). The vascular sensitivity to ACh-induced NO/PGI2-dependent dilatation was not affected by age in DL mice (Table 2). Hence, the following experiments focussed on the impact of age and DL on EDHF-dependent dilatation.
Effect of ageing and DL on cytochrome P450 involvement in EDHF-dependent dilatation to ACh
In arteries isolated from 3 m/o WT mice, 17-ODYA, a cytochrome P450/epoxygenase enzyme inhibitor, had no impact on the dilatation triggered by ACh (Figure 1c, Table 2). In vessels isolated from 12 to 20 m/o WT mice, however, 17-ODYA reduced (P<0.05) ACh-induced EDHF-dependent dilatation (Figure 1c, Table 2).
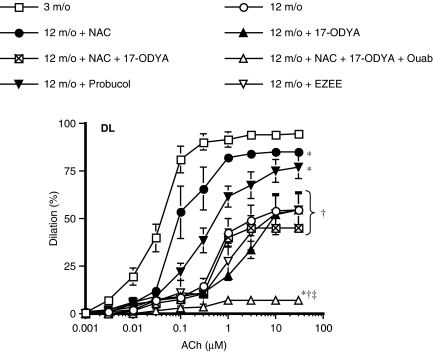
In contrast, 17-ODYA (10 μM) reduced (P<0.05) EDHF-dependent maximal dilatations by 66% in arteries isolated from 3 m/o DL mice (Figure 1d, Table 2). The inhibitory effect of 17-ODYA was lost in arteries isolated from 12 to 20 m/o DL mice (Figure 1d, Table 2). Accordingly, while the EET receptor antagonist EZEE (1 μM) reduced ACh-induced dilatation in 3 m/o DL but not WT mice (Krummen et al., 2005), it no longer reduced the dilatation induced by ACh in vessels isolated from 12 m/o DL mice (Figure 2).
Figure 2.
ACh-induced dilatation of gracilis arteries isolated of 3 and 12 m/o DL mice (n=7 and 8, respectively). In arteries isolated from 12 m/o mice, the effects of NAC (1 μM, n=6), Probucol (10 μM; n=9), EZEE (1 μM), NAC plus 17-ODYA (10 μM, n=6) and NAC plus 17-ODYA and ouabain (1 mM, n=6) are reported. L-NNA (100 μM) and INDO (10 μM) were present in the bath. *P<0.05 compared to 3 m/o; †P<0.05 compared to 12 m/o NAC. ‡P<0.05 compared to 12 m/o 17-ODYA.
Effect of ageing and DL on the involvement of the Na+/K+-ATPase pump and Kir channels in EDHF-dependent dilatation to ACh
Ouabain alone (1 mM), a Na+/K+-ATPase pump inhibitor, strongly reduced (P<0.05) ACh-induced dilatation in arteries isolated from WT mice at all ages (Figure 1e, Table 2). The addition of Ba2+ (30 μM), a Kir channel inhibitor, to ouabain had no impact in arteries isolated from WT mice (data not shown).
In contrast, EDHF-induced dilatation of gracilis arterial segments isolated from 3 m/o DL mice was insensitive to ouabain (Figure 1f, Table 2) At 12 and 20 months of age, however, ouabain strongly reduced (P<0.05) ACh-induced EDHF-dependent dilatation (26±6 and 2±1%, respectively) (Figure 1f, Table 2). The addition of Ba2+ (30 μM) to ouabain reduced (P<0.05) EDHF-dependent maximal dilatation from 95±2 to 54±11% in arteries from 3 m/o DL mice but did not further reduce dilatations induced by EDHF in arteries from 12 m/o mice (26±9%). Ba2+/ouabain experiments were not conducted in arteries from 20 m/o mice since ouabain alone completely prevented ACh-induced EDHF-dependent dilatations (Figure 1f, Table 1).
Effect of ageing and DL on the contribution of small (SKCa) and intermediate (IKCa) conductance calcium-dependant potassium channels in EDHF-dependent dilatation to ACh
Apamin (1 μM), an SKCa channel inhibitor, combined with charybdotoxin (0.1 μM), an IKCa channel inhibitor, reduced by ≈75% the non-NO/non-PGI2 dilatation induced by ACh in arteries isolated from 3 m/o WT and DL mice (Table 2). In vessels from 12 m/o WT mice only, the inhibitory effect of apamin and charybdotoxin was reduced (inhibition of the dilatation by 30%), whereas at 20 months of age, the dilatation was prevented by 75%. In contrast, in DL mice, the combination of the two toxins prevented EDHF-dependent dilatation induced by ACh at 12 and 20 m/o (Table 2).
Effect of ROS on EDHF-dependent dilatation to ACh in ageing and DL
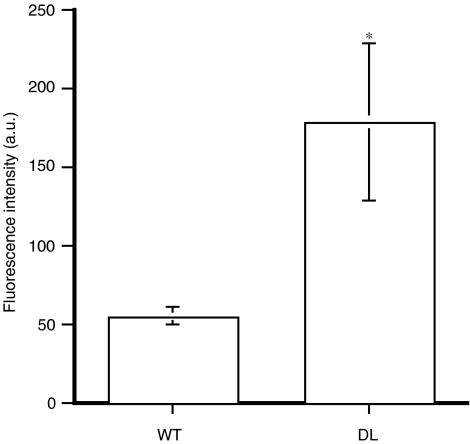
Reactive oxygen species production was increased in arteries isolated from 12 m/o DL mice compared to 12 m/o WT mice (Figure 3). This increased ROS generation was demonstrated in stimulated conditions using angiotensin II (10 μM). NAC (1 μM), a free radical scavenger, did not affect EDHF-induced dilatations of vessels isolated from WT mice (Table 2). Similarly, NAC did not affect the dilatation to ACh of arterial segments isolated from 3 m/o DL mice. At 12 m/o, however, NAC restored (P<0.05) ACh-induced EDHF-dependent dilatation of the DL mouse gracilis artery (increased the dilatation by 47%; Figure 2, Table 2). The beneficial effect of NAC was confirmed using probucol, another free radical scavenger (Figure 2). 17-ODYA (10 μM) abolished (P<0.05) the beneficial effect of NAC on EDHF-dependent dilatations (Figure 2). No effect of NAC could be observed at 20 months of age (Table 1).
Figure 3.
Angiotensin II (10 μM)-induced free radical production of pressurised segments of the gracilis artery isolated from 12 m/o WT and DL mice (n=4). *P<0.05 compared to WT.
Discussion
This study was designed to investigate the effects of DL associated with the ageing process on the nature, efficacy and mechanisms of EDHF-dependent dilatation to ACh of the mouse gracilis artery. EDHF-dependent dilatory responses were observed in the presence of NOS and COX inhibitors (Adeagbo & Triggle, 1993; Brandes et al., 2000; Véquaud & Thorin, 2001).
We previously reported that in the gracilis artery, a resistance vessel, activation of endothelial SKCa and IKCa channels mediate the effects of EDHF. In 3 m/o WT mice, activation of these channels fully dilates smooth muscle cells by activating the Na+/K+-ATPase pump (Krummen et al., 2005). In vessels isolated from DL mice, this pathway contributes to half of the dilatory response to ACh. EETs, derived from the activity of a cytochrome P450 (Krummen et al., 2005), represent a secondary pathway contributing to the dilatation induced by ACh as reported by others (Campbell et al., 1996; Baron et al., 1997; Fisslthaler et al., 1999; Brandes et al., 2000; Archer et al., 2003). The results of the present study demonstrate that over the course of ageing, the functional activity of the cytochrome P450 is inhibited in DL mice due to a rise in oxidative stress, while this pathway becomes expressed in WT mice. At 20 m/o, the activity of the cytochrome P450 remains in WT mice but is irreversibly blunted in DL mice. Hence, the decline in EDHF-dependent dilatation occurring during the normal course of ageing is accompanied by a compensatory duplication of the EDHF pathways; DL hastens the expression of this compensatory process.
Ageing has been reported to be associated with a reduced EDHF-dependent dilatation (Fujii et al., 1993; Urakami-Harasawa et al., 1997; Froese et al., 1999). Our data collected in the gracilis artery are in agreement with these earlier reports. One important finding, however, is the demonstration that the nature of the EDHF released upon muscarinic stimulation is dynamic with age, that is, it is not a single factor that accounts for the dilatation through life. As we reported previously (Krummen et al., 2005), activation of endothelial SKCa and IKCa channels leads to dilatation of the gracilis artery isolated from 3 m/o WT mice. It has been proposed that endothelial SKCa and IKCa are responsible for the rise in [K+]o in the intercellular space (Edwards et al., 1998). This increases the activity of the smooth muscle ouabain-sensitive Na+/K+-ATPase pump leading to hyperpolarisation and dilatation. After 9 months, however, apamin and charybdotoxin only partially reduced the dilatation induced by ACh, suggestive of a change in the endothelial pathways leading to EDHF-mediated dilatation. Since 17-ODYA, a cytochrome P450/epoxygenase inhibitor, reduced the dilatation induced by ACh, it suggests that this pathway is upregulated during the normal ageing process in addition to being activated by DL in young animals.
An important observation is the greater decline in EDHF-mediated dilatation of vessels isolated from DL mice between the age of 3 and 20 months compared to WT. In arteries isolated from 12 m/o DL mice, the activity of the cytochrome P450 pathway no longer contributes to ACh-induced dilatation. The regression of the activity of this pathway is certainly responsible for the sharp drop in EDHF efficacy observed at 12 m/o and further exacerbated at 20 m/o. This P450 pathway, however, has been reported to be sensitive to oxidative stress (Miura & Gutterman, 2004). As demonstrated in Figure 3, the production of ROS is augmented in arteries isolated from 12 m/o DL mice. To validate the potential inactivating role of ROS on the activity of the P450 pathway, we tested the acute effect of NAC and probucol on ACh-induced dilatation of vessels isolated from 12 m/o DL mice (Figure 2). The antioxidants restored the dilatation induced by ACh in these vessels, which was identical to the dilatation induced by ACh in arteries isolated from 12 m/o WT mice (Table 2). This strongly suggests that oxidative stress, which is increased at 12 months in DL (Figure 3), contributes to the decline of the efficacy of EDHF associated with DL. Importantly, addition of 17-ODYA cancelled the protective effect of NAC. Thus, DL-associated ROS production reduces EDHF-dependent dilatation by inhibiting the cytochrome P450 function and EET production. Hence, DL favours the early expression of a secondary EDHF pathway, a pathway that only appears at 12 months of age in WT mice. DL, however, is associated with a rise in oxidative stress, which is responsible for the functional inactivation of this early compensatory pathway.
The signals leading to the duplication of the EDHF pathways are unknown. NAC only improved dilatation of vessels isolated from DL mice to normal WT mice levels. In contrast, NAC did not magnify the dilatation in WT mice. It suggests that the NAC-sensitive oxidative stress per se is not responsible for the upregulation of this cytochrome P450 pathway. It is also unlikely that maturation alone is responsible for the late expression of the cytochrome P450 pathway in WT mice since this expression is hastened by DL. Hence, the expression of this pathway must be sensitive either to the functional status of the endothelium or to the extracellular matrix environment.
The mechanisms underlying EDHF-induced dilatation are indeed different at 3 m/o in DL and WT mice (Krummen et al., 2005). Whereas the sensitivity to apamin and charybdotoxin is identical, ouabain did not prevent dilatation of arteries isolated from DL mice. At 12 m/o, however, the contribution of these signalling proteins changed: the inhibitory effects of apamin and charabdotoxin on the dilatation induced by ACh in WT mice is reduced while the combination of the toxins prevented the dilatory response in DL mice. In both groups, however, the toxins augmented the myogenic tone (Table 1), suggesting that the KCa channels are contributing to the baseline regulation of smooth muscle contractility. Similarly, ouabain reduced the dilatation induced by ACh in both groups of 12 m/o animals, while myogenic tone was augmented by ouabain at all ages for both groups. Hence, the basal activity of the endothelial KCa channels and the smooth muscle Na+/K+-ATPase are similar in arteries isolated from WT and DL mice, if one uses the myogenic tone (baseline diameter) as an index, whereas upon muscarinic receptor stimulation discrepancies appear. This, therefore, is more suggestive of an impaired receptor/effector coupling rather than a fundamental change in the endothelial function.
This does not eliminate the possibility that the extracellular environment is altered by the ageing process and exacerbated by DL. It is known that ageing is associated with intimal thickening of the vessels (Stary, 1990). In this condition, coupling between endothelial and smooth muscle cells is expected to be impaired, and the hyperpolarising diffusion of K+ ions from the endothelium to the smooth muscle cells, as proposed by our data and Edwards and co-workers (1998) would be reduced. This is, however, unlikely because the histological analysis did not reveal changes in the structure of the gracilis arteries (data not shown). In addition, blockade of KCa channels by the toxins or the Na+/K+-ATPase with ouabain reduced the resting diameter at all ages (Table 1). These mechanisms remain therefore effective. In addition, we previously reported that gap junction were not involved in the mediation of the effects of EDHF at 3 months (Krummen et al., 2005).
Finally, ageing affected NO/PGI2-dependent dilatation similarly whether arteries were isolated from WT or DL mice. This age-dependent endothelial dysfunction is well established (Gerhard et al., 1996; Rodriguez-Martinez et al., 1998; van der Loo et al., 2000; Woodman et al., 2003). In a recent study, it was reported that the NO-dependent dysfunction could be restored by acute exposure to a SOD mimetic (Csiszar et al., 2002). It is, therefore, likely that in our model too the decline in this function is concomitant with a chronic rise in oxidative stress.
In conclusion, a 17-ODYA-sensitive metabolite of the cytochrome P450/epoxygenase pathway, most likely EETs, and KCa channels are responsible for ACh-induced EDHF-dependent dilatation of the DL mouse gracilis artery. At 12 m/o, DL targets the 17-ODYA-sensitive component of the dilatation mediated by ACh, as revealed by the rescue of this dilatation by antioxidants, whereas this 17-ODYA-sensitive mechanism appears in 12 m/o WT mice. Free radical production was therefore responsible for this early – but reversible – endothelial dysfunction in DL mice. At 20 months of age, NAC no longer rescued the dilatation suggestive of an irreversible damage to the endothelium. Hence, the 17-ODYA-dependent dilatation is a secondary endothelium-dependent dilatory mechanism recruited early in the presence of a risk factor for CVDs such as DL and latter in life during the normal ageing process. This time-dependent evolution of the endothelial function suggests that there is a therapeutic window for the prevention of the endothelial dysfunction associated with DL. Because of the sensitivity of EDHF to oxidative stress, antioxidant therapeutic agents may be of use in the early phases of DL and later in life to prevent the deterioration of the endothelial dysfunction, although the impact of oxidative stress to age-associated endothelial dysfunction needs further exploration.
Acknowledgments
This work has been supported in part by the Foundation of the Montreal Heart Institute, the Heart and Stroke Foundation of Quebec and the Canadian Institute for Health Research (MOP14496), the NIH (GM31278, JRF) and the Robert A. Welch Foundation (JRF). E. Thorin is a senior scholar of the Fonds de la Recherche en Santé du Québec.
Abbreviations
- ACh
acetylcholine
- Apa
apamin
- Ba2+
barium
- Chtx
charybdotoxin
- DL
dyslipidaemia
- Emax
maximal dilatation
- EDHF
endothelium-derived hyperpolarising factor
- EETs
epoxyecosatrienoic acids
- EEZE
14,15-epoxyeicosa-5(Z)-enoic acid
- INDO
indomethacin
- KCa
Ca2+-sensitive K+ channel
- Kir
inward rectifier potassium channels
- L-NNA
Nω-nitro-L-arginine
- MT
myogenic tone
- NAC
N-acetyl-L-cysteine
- NO
nitric oxide
- Ouab
ouabain
- PE
phenylephrine
- PGI2
prostacyclin
- PSS
physiological salt solution
- SNP
sodium nitroprusside
- 17-ODYA
17-octadecynoic acid
References
- ADEAGBO A.S., TRIGGLE C.R. Varying extracellular [K+]: a functional approach to separating EDHF and EDNO related mechanisms in perfused rat mesenteric arterial bed. J. Cardiovasc. Pharmacol. 1993;21:423–429. [PubMed] [Google Scholar]
- ARCHER S.L., GRAGASIN F.S., WU X., WANG S., MCMURTRY S., KIM D.H., PLATONOV M., KOSHAL A., HASHIMOTO K., CAMPBELL W.B., FALCK J.R., MICHELAKIS E.D. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BK(Ca) channels. Circulation. 2003;107:769–776. doi: 10.1161/01.cir.0000047278.28407.c2. [DOI] [PubMed] [Google Scholar]
- BARON A., FRIEDEN M., BENY J.L. Epoxyeicosatrienoic acids activate a high-conductance, Ca2+-dependent K+ channel on pig coronary artery endothelial cells. J. Physiol. 1997;504:537–543. doi: 10.1111/j.1469-7793.1997.537bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRANDES R.P., BEHRA A., LEBHERZ C., BOGER R.H., BODE-BOGER S.M., PHIVTHONG-NGAM L., MUGGE A. N(G)-nitro-L-arginine- and indomethacin-resistant endothelium-dependent relaxation in the rabbit renal artery: effect of hypercholesterolemia. Atherosclerosis. 1997;135:49–55. doi: 10.1016/s0021-9150(97)00145-7. [DOI] [PubMed] [Google Scholar]
- BRANDES R.P., SCHMITZ-WINNENTHAL F.H., FELETOU M., GODECKE A., HUANG P.L., VANHOUTTE P.M., FLEMING I., BUSSE R. An endothelium-derived hyperpolarizing factor distinct from NO and prostacyclin is a major endothelium-dependent vasodilator in resistance vessels of wild-type and endothelial NO synthase knockout mice. Proc. Natl. Acad. Sci. U.S.A. 2000;97:9747–9752. doi: 10.1073/pnas.97.17.9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUSSEMAKER E., POPP R., FISSLTHALER B., LARSON C.M., FLEMING I., BUSSE R., BRANDES R.P. Aged spontaneously hypertensive rats exhibit a selective loss of EDHF-mediated relaxation in the renal artery. Hypertension. 2003;42:562–568. doi: 10.1161/01.HYP.0000088852.28814.E2. [DOI] [PubMed] [Google Scholar]
- CAMPBELL W.B., GEBREMEDHIN D., PRATT P.F., HARDER D.R. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ. Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- CHAYTOR A.T., EDWARDS D.H., BAKKER L.M., GRIFFITH T.M. Distinct hyperpolarizing and relaxant roles for gap junctions and endothelium-derived H2O2 in NO-independent relaxations of rabbit arteries. Proc. Natl. Acad. Sci. U.S.A. 2003;100:15212–15217. doi: 10.1073/pnas.2435030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREAGER M.A., COOKE J.P., MENDELSOHN M.E. Impaired vasodilatation of forearm resistance vessels in hypercholesterolemic humans. J. Clin. Invest. 1990;86:228–234. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSISZAR A., UNGVARI Z., EDWARDS J.G., KAMINSKI P., WOLIN M.S., KOLLER A., KALEY G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ. Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- DORA K.A., SANDOW S.L., GALLAGHER N.T., TAKANO H., RUMMERY N.M., HILL C.E., GARLAND C.J. Myoendothelial gap junctions may provide the pathway for EDHF in mouse mesenteric artery. J. Vasc. Res. 2003;40:480–490. doi: 10.1159/000074549. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., DORA K.A., GARDENER M.J., GARLAND C.J., WESTON A.H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- FISSLTHALER B., POPP R., KISS L., POTENTE M., HARDER D.R., FLEMING I., BUSSE R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- FLAVAHAN N.A. Atherosclerosis of lipoprotein-induced endothelial dysfunction: potential mechanisms underlying reduction in EDRF/nitric oxide activity. Circulation. 1992;85:1927–1938. doi: 10.1161/01.cir.85.5.1927. [DOI] [PubMed] [Google Scholar]
- FROESE D.E., MCMASTER J., MAN R.Y., CHOY P.C., KROEGER E.A. Inhibition of endothelium-dependent vascular relaxation by lysophosphatidylcholine: impact of lysophosphatidylcholine on mechanisms involving endothelium-derived nitric oxide and endothelium-derived hyperpolarizing factor. Mol. Cell. Biochem. 1999;197:1–6. doi: 10.1023/a:1006847929334. [DOI] [PubMed] [Google Scholar]
- FUJII K., OHMORI S., TOMINAGA M., ABE I., TAKATA Y., OHYA Y., KOBAYASHI K., FUJISHIMA M. Age-related changes in endothelium-dependent hyperpolarization in the rat mesenteric artery. Am. J. Physiol. 1993;265:509–516. doi: 10.1152/ajpheart.1993.265.2.H509. [DOI] [PubMed] [Google Scholar]
- GERHARD M., RODDY M.A., CREAGER S.J., CREAGER M.A. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of human. Hypertension. 1996;27:849–853. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- KOLODGIE F.D., VIRMANI R., RICE H.E. Vascular reactivity during the pro-gresssion of atherosclerotic plaque. A study of Watanabe heritable hyperlipidemic rabbits. Circ. Res. 1990;66:1112–1126. doi: 10.1161/01.res.66.4.1112. [DOI] [PubMed] [Google Scholar]
- KRUMMEN S., FALCK J.R., THORIN E. Two pathways account for EDHF-dependent dilation in the gracilis artery of hypercholesterolemic hApoB+/+ mice. Br. J. Pharmacol. 2005;145:264–270. doi: 10.1038/sj.bjp.0706194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARIN J. Age-related changes in vascular responses: a review Mech. Ageing Dev. 1995;79:71–114. doi: 10.1016/0047-6374(94)01551-v. [DOI] [PubMed] [Google Scholar]
- MIURA H., GUTTERMAN D.D.Interaction of two distinct endothelium-derived hyperpolarizing factors in the human coronary circulation Circulation 2004110III-181(abstract) [Google Scholar]
- MIURA H., BOSNJAK J.J., NING G., SAITO T., MIURA M., GUTTERMAN D.D. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ. Res. 2003;92:e31–e40. doi: 10.1161/01.res.0000054200.44505.ab. [DOI] [PubMed] [Google Scholar]
- MULLER-DELP J.M., SPIER S.A., RAMSEY M.W., DELP M.D. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am. J. Physiol. 2002;283:H1662–H1672. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- NAGAO T., ILLIANO S.C., VANHOUTTE P.M. Heterogeneous distribution of endothelium-dependent relaxations resistant to nitro-L-arginine in the arterial tree of the rat. Am. J. Physiol. 1992;263:H1090–H1094. doi: 10.1152/ajpheart.1992.263.4.H1090. [DOI] [PubMed] [Google Scholar]
- NAJIBI S., COWAN C.L., PALACINO J.J., COHEN R.A. Enhanced role of potassium channels in relaxations to acetylcholine in hypercholesterolemic rabbit carotid artery. Am. J. Physiol. 1994;266:H2061–H2067. doi: 10.1152/ajpheart.1994.266.5.H2061. [DOI] [PubMed] [Google Scholar]
- NGUYEN T.D., VEQUAUD P., THORIN E. Effects of endothelin receptor antagonists and nitric oxide on myogenic tone and alpha-adrenergic-dependent contractions of rabbit resistance arteries. Cardiovasc. Res. 1999;43:755–761. doi: 10.1016/s0008-6363(99)00170-4. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ-MARTINEZ M.A., ALONSO M.J., REDONDO J., SALAICES M., MARIN J. Role of lipid peroxidation and the glutathione-dependent antioxydant system in the impairment of endothelium-dependent vasodilations with age. Br. J. Pharmacol. 1998;123:113–121. doi: 10.1038/sj.bjp.0701595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANAN D.A., NEWLAND D.L., TAO R., MARCOVINA S., WANG J., MOOSER V., HAMMER R.E., HOBBS H.H. Low density lipoprotein receptor-negative mice expressing human apolipoprotein B-100 develop complex atherosclerotic lesions on a chow diet: no accentuation by apolipoprotein(a) Proc. Natl. Acad. Sci. U.S.A. 1998;95:4544–4549. doi: 10.1073/pnas.95.8.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STARY H.C. The sequence of cell and matrix changes in atherosclerotic lesions of coronary arteries in the first forty years of life. Eur. Heart J. 1990;11 Suppl E:3–19. doi: 10.1093/eurheartj/11.suppl_e.3. [DOI] [PubMed] [Google Scholar]
- URAKAMI-HARASAWA L., SHIMOKAWA H., NAKASHIMA M., EGASHIRA K., TAKESHITA A. Importance of endothelium-derived hyperpolarizing factor in human arteries. J. Clin. Invest. 1997;100:2793–2799. doi: 10.1172/JCI119826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER LOO B., LABUGGER R., SKEPPER J.N., BACHSCHMID M., KILO J., POWELL J.M., PALACIOS-CALLENDER M., ERUSALIMSKY J.D., QUASCHNING T., MALINSKI T., GYGI D., ULLRICH V., LUSCHER T.F. Enhanced peroxynitrite formation is associated with vascular aging. J. Exp. Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANHOUTTE P.M., SCOTT-BURDEN T. The endothelium in health and disease. Texas Heart Inst. J. 1994;21:62–67. [PMC free article] [PubMed] [Google Scholar]
- VÉQUAUD P., THORIN E. Endothelial G protein beta-subunits trigger nitric oxide-but not endothelium-derived hyperpolarizing factor-dependent dilation in rabbit resistance arteries. Circ. Res. 2001;89:716–722. doi: 10.1161/hh2001.097783. [DOI] [PubMed] [Google Scholar]
- WOODMAN C.R., PRICE E.M., LAUGHLIN M.H. Selected contribution: aging impairs nitric oxide and prostacyclin mediation of endothelium-dependent dilation in soleus feed arteries. J. Appl. Physiol. 2003;95:2164–2170. doi: 10.1152/japplphysiol.01073.2002. [DOI] [PubMed] [Google Scholar]