Abstract
GPRC6A is a novel family C G-protein-coupled receptor (GPCR) with so far unknown physiological function. It was the aim of our study to further characterize the ligand preferences of the receptor and elucidate structural requirements for activity.
We have previously generated a functional chimeric receptor construct, h6A/5.24, containing the ligand-binding amino-terminal domain of the human GPRC6A and the seven-transmembrane domain and carboxy terminus of the homologous goldfish receptor 5.24. Based on knowledge that this chimera prefers basic L-α-amino acids such as arginine, lysine and ornithine, we searched for commercially available analogues of these and other L-α-amino acids, and tested them for activity in a fluorescence-based calcium assay. The majority of the tested compounds are involved in the regulation of nitric oxide synthase (NOS) and arginase enzymes. Altogether we profiled 30 different analogues.
We found that a structurally wide range of L-α-amino-acid analogues of both arginine, lysine, and ornithine are agonists at h6A/5.24, whereas no antagonists were identified. From the profiling it is concluded that L-α-amino acids containing a highly basic side chain confer the highest activity, although the most potent compound was only twice as potent as L-arginine, which has a EC50 value of 23.5 μM.
The reported agonism of NOS- and arginase-active compounds at GPRC6A has obvious implications in interpretation of experiments involving the NOS and arginase systems, and interfering effects at GPRC6A should be regarded of relevance, especially as the physiological function of the receptor is not yet understood.
Keywords: Arginase, G-protein-coupled receptor, GPRC6A, h6A/5.24, L-α-amino-acid analogues, nitric oxide synthase
Introduction
The G-protein-coupled receptor, family C, group 6, subtype A (GPRC6A) is a novel member of family C of G-protein-coupled receptors (GPCRs) characterized by a large extracellular amino-terminal domain containing the endogenous ligand-binding region, a cysteine-rich region, and a seven-transmembrane domain involved in the G-protein coupling (Pin et al., 2003; 2004). GPRC6A shares the highest amino-acid sequence identity (45%) with the goldfish odorant receptor 5.24 (Speca et al., 1999), and displays 24–34% sequence identity with the calcium-sensing receptor (Brown et al., 1993), the T1R subtype 1 taste receptor (Nelson et al., 2001; 2002), and the metabotropic glutamate (mGlu) subtype 1 receptor (Hermans & Challiss, 2001).
GPRC6A appears to be conserved through evolution. Among mammals, both human and mouse orthologues have been cloned and characterized (Wellendorph & Bräuner-Osborne, 2004; Kuang et al., 2005; Wellendorph et al., 2005). The GPRC6A gene is composed of six exons and alternative splicing between these renders at least three isoforms of different length and abundance. In both species, the longest and most abundant isoform is widely distributed and expressed in similar tissues. Furthermore, based on a pronounced sequence and ligand preference similarity to the goldfish 5.24 and the zebrafish ZO6, these receptors are most likely orthologues of GPRC6A (Speca et al., 1999; Kuang et al., 2003; Luu et al., 2004).
As reported earlier, the wild-type human GPRC6A is not expressed on the surface of cell lines, indicating that scaffolding and/or interacting proteins are needed for processing and trafficking of the receptor protein (Wellendorph & Bräuner-Osborne, 2004). To overcome this obstacle we used a chimeric receptor consisting of the amino-terminal domain of GPRC6A and the signalling domain of the goldfish receptor 5.24 (h6A/5.24), which is surface expressed and couples to Gαq to deorphanize the receptor (Wellendorph et al., 2005). The deorphanization revealed that L-α-amino acids, and in particular the basic amino acids L-arginine, L-lysine, and L-ornithine, act as endogenous agonists at the receptor (Wellendorph et al., 2005). This has provided the first clues as to the physiology of the receptor, and following, several hypotheses about the function of GPRC6A have been put forward, which also reflect the wide tissue distribution. The finding that the most potent agonists are intermediates of the urea cycle, could infer that the receptor is linked to this regulatory pathway. Based on the homology to the calcium-sensing receptor, and the known physiological role of this receptor, GPRC6A has also been suggested to be a sensor of the free amino-acid concentration in the blood. Alternatively GPRC6A could represent a novel receptor relevant to the nervous system (Wellendorph et al., 2005). Other postulated roles include monitoring of cell death (Civelli, 2005), partaking in a novel cellular communication system (Kuang et al., 2005) or extracellular sensing of divalent cations (Pi et al., 2005).
In order to further clarify the pharmacology and physiological role of GPRC6A, one major objective is to identify selective and potent agonists and antagonists that can serve as investigational tools or as lead structures for further ligand development. Seeing that GPRC6A is a rather promiscuous -amino-acid receptor we searched for commercially available analogues of L-arginine, L-lysine, and L-ornithine of which several are described as regulators of nitric oxide synthase (NOS) (Southan & Szabó, 1996; Luzzi & Marletta, 2005; Proskuryakov et al., 2005) or arginase (Robertson et al., 1993; Christianson, 2005). L-Arginine is namely also a substrate for NOS, leading to the generation of nitric oxide (NO), an important signalling, immuno-, and vasoreactive molecule (Böger & Bode-Böger, 2001), and for arginase in the urea cycle, responsible for converting L-arginine to L-ornithine and urea (Christianson, 2005). We hypothesized that these known L-α-amino-acid analogues would act at GPRC6A, either as agonists or antagonists. Investigation of this hypothesis is relevant for gaining further insight into the structure–activity relations of GPRC6A, but also for testing the specificity of regulators applied in NOS and arginase research.
Methods
Cell culturing and transfection
tsA201 cells (a transformed HEK293 cell line) (Chahine et al., 1994) were cultured in GlutaMAX-I Dulbecco's-modified Eagle's medium supplemented with 10% dialysed foetal bovine serum, penicillin (100 U ml−1), and streptomycin (100 mg ml−1) at 37°C in a humidified atmosphere of 95% air and 5% CO2.
The chimeric receptor construct, h6A/5.24, consisting of the amino-terminal domain of human GPRC6A and the seven-transmembrane domain and carboxy terminus of the goldfish receptor 5.24 has previously been described (Wellendorph et al., 2005). For transient expression, this construct was transfected into tsA201 cells using PolyFect according to the manufacturer's protocol (Qiagen, West Sussex, U.K.).
Fluorescence-based measurement of intracellular calcium levels
tsA cells transiently expressing h6A/5.24 were split into poly-D-lysine-coated black 96-well plates with clear bottoms (BD Biosciences, Palo Alto, CA, U.S.A.) as previously described (Wellendorph et al., 2005). Determination of activity of diverse analogues of basic L-α-amino acids at the receptor was performed by measurement of intracellular calcium levels as described by Kuang et al. (2003) and Wellendorph et al. (2005). This method was chosen because h6A/5.24 can activate the goldfish receptor 5.24-dependent signal transduction pathway, that is, the coupling to Gαq and thereby the stimulation of phospholipase C, the production of inositol 1,4,5-trisphosphate, and the release of intracellular calcium (Speca et al., 1999; Kuang et al., 2003). Furthermore, compared to, for example assays measuring phosphoinositol turnover this assay system is simple, robust, and nonradioactive.
In brief, cells were washed with assay buffer (Hanks' balanced saline solution supplemented with 20 mM HEPES, 1 mM CaCl2, 1 mM MgCl2, and 1 mg ml−1 BSA, pH 7.4) and preincubated for 2 × 2 h in 100 μl assay buffer. The cells were then incubated for 1 h with 50 μl assay buffer containing 6 μM Fluo-4AM and 2.5 mM probenecid. Finally, cells were washed three times with 100 μl final assay buffer (Hanks' balanced saline solution supplemented with 20 mM HEPES, 1 mM CaCl2, 1 mM MgCl2, and 2.5 mM probenecid, pH 7.4) and incubated with 150 μl final assay buffer for 30 min. All incubations were at 37°C.
Stock solutions of all compounds were made in water at 100 mM except L-arginine β-naphthylamide (0.15 N NaOH), L-S-[2-(4-pyridyl)ethyl]-cysteine (0.1 N NaOH) and L-NG-nitroarginine (L-NOARG) (0.1 N HCl). All compounds were tested at eight different concentrations ranging from approximately 350 nM to 1 mM. In case of inactivity, compounds were tested for antagonism of the response mediated by submaximal concentration of L-arginine (90 μM).
Responses were measured in a NOVOstar microplate reader using excitation/emission wavelengths of 485/520 nm. All experiments were performed in triplicate and repeated in at least three independent experiments.
Materials
GlutaMAX-I Dulbecco's-modified Eagle's medium, dialysed foetal bovine serum, penicillin, streptomycin, Hanks' balanced saline solution, and BSA were all from Invitrogen (Paisley, U.K.). PolyFect was obtained from Qiagen (West Sussex, U.K.).
L-NG-Hydroxyarginine (L-NOHA), L-canavanine, L-arginine, L-homoarginine, L-NOARG, asymmetric L-NG,NG-dimethylarginine (ADMA), L-NG-nitroarginine methyl ester (L-NAME), L-ornithine, DL-5-hydroxylysine, L-N6-(1-iminoethyl)-lysine (L-NIL), L-lysine, L-S-(2-aminoethyl)-cysteine, L-S-[2-(4-pyridyl)ethyl]-cysteine, L-citrulline, L-N-α-acetylarginine, L-arginine β-naphthylamide, L-arginine hydroxamate, L-carnosine, DL-α-difluoromethyl-ornithine (DFMO), L-β-homolysine, L-3-(3-pyridyl)-alanine, agmatine, aminoguanidine, L-norleucine, L-norvaline, Nɛ,Nɛ,Nɛ-trimethyllysine, probenecid, and all buffer reagents were purchased from Sigma-Aldrich (St Louis, MO, U.S.A.). L-NG-Mono-methylarginine (L-NMMA), L-NG-propylarginine, and L-N5-(1-iminoethyl)-ornithine (L-NIO) were purchased from Tocris Bioscience (Ellisville, MO, U.S.A.). L-NG-Aminoarginine, L-NG-hydroxy-nor-arginine (L-nor-NOHA), L-NG-mono-methylhomoarginine (L-NMMHA), L-N5-(1-imino-3-butenyl)-ornithine (vinyl-L-NIO), and L-thiocitrulline were purchased from Alexis Biochemicals (Lausen, Switzerland). Fluo-4AM was obtained from Molecular Probes (Eugene, OR, U.S.A.).
Data analysis
Responses were calculated as peak fluorescence after agonist addition subtracted fluorescence before agonist addition. Concentration–response curves were analysed by nonlinear regression using Prism 4.0b (GraphPad Software, San Diego, CA, U.S.A.). The maximum concentration used for generation of concentration–response curves was the concentration at which no significant activity was observed on mock-transfected cells. Owing to low potency or significant effect on mock-transfected cells at high concentrations, some compounds were tested at submaximal concentrations. Under the assumption that these compounds were full agonists at the receptor, concentration–response curves were fitted to the maximum response of L-arginine, which was always included as a control.
Results
In the hope to identify novel potent ligands and generate a broader structure–activity relationship of compounds interacting with GPRC6A, we wished to elaborate on the series of already tested naturally occurring amino acids. Given previous ligand characterization and homology modelling studies showing that the human GPRC6A interacts stereoselectively with L-α-amino acids, and preferentially those containing a positively charged side chain (Wellendorph et al., 2005), we decided to focus primarily on basic amino acids. Accordingly, the majority of the tested analogues in this work are derivatives of L-arginine, modified at the distal guanidinium group or diverse amino acids carrying different distal substituents. Many of the commercially available compounds of this sort are primarily known for their inhibitory or substrate-like activities on various NOS and arginase enzymes (Table 1). However, to attain even more diversity, a few additional compounds, with no reported activity at these enzymes, were purchased and included in the study as well.
Table 1.
NOS and arginase substrates and inhibitors tested for activity at h6A/5.24
| NOS substratesa |
| L-Arginine (natural substrate), L-homoarginine, L-NOHA, L-canavanineb |
| NOS inhibitorsc |
| ADMA, aminoguanidine, L-NG-aminoarginine, L-canavanine, L-NAME, L-NMMA, L-NMMHA, L-NOARG, L-NG-propylarginine, L-NIL, L-NIO, vinyl-L-NIO, L-thiocitrulline |
| Arginase substratesd |
| Agmatine, L-arginine (natural substrate), L-canavanine, L-homoarginine |
| Arginase inhibitorsd |
| L-NOARGe, L-NOHA, L-nor-NOHA |
Taken from Luzzi & Marletta (2005) and Southan & Szabó (1996).
Reported as a weak NOS substrate (Yokoi et al., 1994).
Taken from Proskuryakov et al. (2005) and Southan & Szabó (1996).
Taken from Christianson (2005).
Reported as rat liver arginase inhibitor Robertson et al. (1993).
Compounds were tested for their ability to increase intracellular calcium levels in cells transiently expressing the chimera h6A/5.24 in response to activation of the receptor (as described in Methods). Concentration–response curves were generated to determine the potency of the compounds (Figure 1). In general the results show that all tested basic L-α-amino acids containing an intact glycine moiety are agonists at h6A/5.24 (Tables 2 and 3), whereas compounds modified at the glycine moiety are devoid of any agonist or antagonist activity (Table 4).
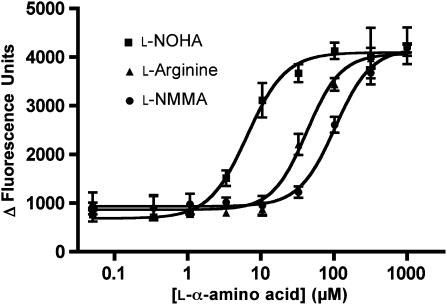
Figure 1.
Representative concentration–response curves of L-arginine and the two L-arginine analogues L-NG-hydroxyarginine (L-NOHA), and L-NG-mono-methylarginine (L-NMMA) at h6A/5.24. Data were obtained by intracellular calcium measurements using the calcium-sensitive dye Fluo-4 in living tsA cells transiently expressing the chimeric receptor construct h6A/5.24. Responses are shown as Δ fluorescence units (peak fluorescence after agonist addition subtracted fluorescence before agonist addition). Data shown are mean±s.d. of a single experiment performed in triplicate. EC50 values for all analogues are shown in Tables 2 and 3.
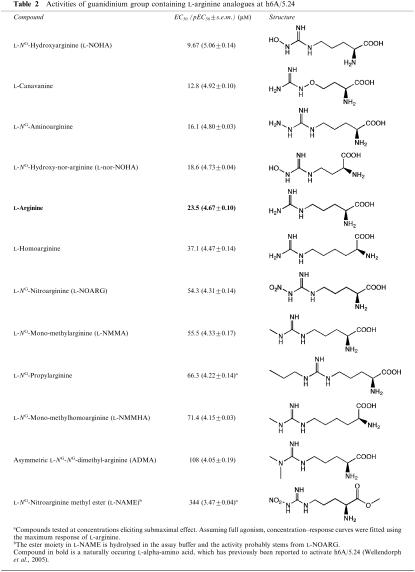
Table 2.
Activities of guanidinium group containing L-arginine analogues at h6A/5.24
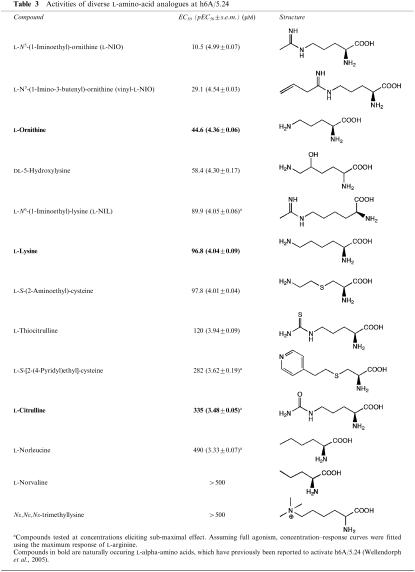
Table 3.
Activities of diverse L-amino-acid analogues at h6A/5.24
Table 4.
Structures of L-amino-acid analogues inactive at h6A/5.24
Among the guanidinium group containing L-arginine analogues (Table 2), four compounds, L-NOHA, L-canavanine, L-NG-aminoarginine, and L-nor-NOHA, displayed potencies similar to or higher than L-arginine, although the maximum gain in potency was only of a factor two. L-NG-Aminoarginine and L-nor-NOHA were equipotent with L-arginine. Analogues with an alkylated guanidinium group were generally weaker agonists than L-arginine, as exemplified by L-NMMA, L-NG-propylarginine, and ADMA. L-Homoarginine which contains one more carbon than L-arginine in the backbone, was approximately equipotent with L-arginine, while the corresponding methylated guanidinium analogue, L-NMMHA, showed reduced activity at the receptor. Interestingly, for L-NAME, a methyl ester of L-NOARG, an EC50 of 344 μM was obtained suggesting that this compound displays some activity despite the modification at the glycine moiety. However, it was subsequently discovered that L-NAME hydrolyses quite rapidly to L-NOARG in the assay buffer used (up to 20% degradation over the course of the assay; data not shown). Thus, the measured activity probably stems from L-NOARG (EC50 of 54.3 μM) and not L-NAME (Table 2).
Of the tested diverse amino acids, L-NIO was approximately four times more potent than L-ornithine and twice as potent as L-arginine, while the closely related vinyl-L-NIO displayed a potency comparable to L-arginine, and DL-5-hydroxylysine was almost twice as potent as the parent L-lysine. The iminoethyl analogue, L-NIL, with one more carbon than L-NIO in the backbone, was equipotent with L-lysine and approximately three times less potent than the corresponding L-homoarginine. An N-methylated analogue of lysine, Nɛ,Nɛ,Nɛ-trimethyllysine, showed only weak activity at the receptor. The non-basic L-thiocitrulline and L-citrulline, in which the guanidinium group of L-arginine is exchanged for a thioamide or amide function, respectively, displayed markedly weaker activity at the receptor than L-arginine. Two commercially available L-cysteine analogues, L-S-(2-aminoethyl)-cysteine, and L-S-[2-(4-pyridyl)ethyl]cysteine) as well as two compounds lacking the amino group in the side chain, L-norleucine, and L-norvaline, were also examined but yielded no gain of potency (Table 3).
When the distal guanidinium group is intact as in L-arginine, but the proximal glycine modified, compounds were generally devoid of any agonist or antagonist activity. This is exemplified by L-N-α-acetylarginine, agmatine, aminoguanidine, L-arginine β-naphtylamide, and L-arginine hydroxamate, all of which were inactive both as agonists and antagonists. Other amino acids at which the basic side chain is intact but the glycine moiety modified, were similarly inactive at the receptor (examples include L-carnosine and the L-lysine analogues DFMO and β-homolysine). Finally, a weakly basic analogue of L-phenylalanine, L-3-(3-pyridyl)-alanine, was tested, but also displayed no activity (Table 4).
Discussion
We recently uncovered GPRC6A as a promiscuous L-α-amino-acid-sensitive receptor (Wellendorph et al., 2005). With this study, we extend the molecular pharmacology of the receptor beyond the interaction with the naturally occurring amino acids, and show that commercially available L-α-amino-acid analogues of arginine, lysine, and ornithine are agonists at the human GPRC6A. From prior homology modelling studies of GPRC6A (Wellendorph et al., 2005) and homologous receptors from gold- and zebrafish (Kuang et al., 2003; Luu et al., 2004), it stands clear that certain signature residues known from other family C GPCRs (Bertrand et al., 2002) to interact with the glycine moiety of amino acids, are also present in these receptors. Although the current pharmacology was measured on a chimeric h6A/5.24 receptor, previous mutational studies have confirmed that L-arginine binds to the predicted binding pocket, situated within the amino-terminal domain of GPRC6A (Wellendorph et al., 2005). Binding studies on the homologous goldfish 5.24 receptor have furthermore demonstrated that the arginine analogue L-homoarginine can compete with L-arginine binding (Speca et al., 1999). Hence, it is highly conceivable that the analogues in this work bind to the human GPRC6A inherent amino-terminal domain and not to other parts of the chimera.
We have previously characterized a number of naturally occurring L-α-amino acids as agonists at GPRC6A (Wellendorph et al., 2005), and we now demonstrate that derivatives of basic amino acids are also agonists at GPRC6A. From the current data, important information about the structure–activity relationship of compounds interacting with GPRC6A can be deduced. The current work indicates that the structural requirements at the distal end of the binding pocket, which is expected to coordinate to the distal end of the amino acid, are rather loose. This is reflected in the general finding that L-α-amino acids with a basic side chain are all agonists at the human GPRC6A and the array of EC50 values is quite narrow. A few findings and trends are, however, noteworthy. Five of the tested L-amino-acid analogues displayed potencies similar to or higher than L-arginine, which is the most potent of the classical L-α-amino acids. Although the maximum gain in potency was only two-fold, these compounds are the most active GPRC6A agonists reported to date, and represent novel lead structures. For the distal basic substituent, an iminoethyl (as in L-NIO) confers higher activity than a guanidinium (as in L-arginine) or a primary amino group (as in L-ornithine). This reflects that both steric factors and basicity are important, which is further substantiated by the low potency of the positively charged quaternary amine, Nɛ,Nɛ,Nɛ-trimethyllysine. The lack of direct correlation between basicity of the distal moiety and potency is also seen by the rank order of potency of L-NOHA (slightly less basic than L-arginine)>L-arginine>L-NOARG (less basic than L-arginine)=L-NMMA (more basic than L-arginine). Thus, other factors than size and basicity, such as the possibility of making additional hydrogen bonds, influence potency. The corresponding set of compounds, L-lysine and L-NIL, were equipotent but much less potent than L-ornithine and L-NIO, leading to the speculation that the number of carbons in the backbone is approximating the limit of acceptance in the receptor-binding pocket. However, the potency of L-homoarginine when compared to L-arginine is not affected nearly as much as would be expected from the corresponding L-NIL/L-NIO set of compounds, altogether suggesting that strong basic properties of a compound is the dominating factor of importance. This tendency is further substantiated by the low activities obtained for both the two analogues lacking the distal amino group and the two L-α-amino analogues containing weakly basic pyridyl groups, indicating that a pyridyl function is not a suitable bioisostere for the more basic distal guanidinium in L-arginine or the primary amine in L-ornithine/L-lysine. Therefore, a general trend for GPRC6A agonism is that activity is higher when a polar, basic group rather than a nonpolar, aromatic, or nonbasic group is substituted at the distal end of the amino acid.
By contrast, the requirements to the proximal end of the ligand, proposed to interact with the proximal part of the GPRC6A-binding pocket, are much more restricted, as both stereochemistry and substitution are highly critical for activity. So far, no compounds devoid of an L-α-amino-acid moiety have been found capable of activating or inhibiting GPRC6A, which correlates well with structure–activity studies conducted on the homologous goldfish 5.24 receptor (Speca et al., 1999). Actually, none of the currently tested compounds substituted at the glycine moiety affords a GPRC6A ligand. This is interesting compared to the mGlu receptor field, where antagonists can be derived from agonists by relatively small structural changes to glutamate, such as α-substitution of the glycine moiety (Bräuner-Osborne et al., 2000).
Crystal structures of the extracellular glutamate-binding domain of the homodimeric mGlu1 receptor have demonstrated that receptor activation is dependent upon closure of at least one of the two venus fly trap domains, which then stabilizes the closed conformation, and drives the dimer into the active state (Kunishima et al., 2000; Tsuchiya et al., 2002). Agonists stimulate this domain closure. Accordingly, it has been shown that competitive antagonists hinder closure and thus prevent receptor activation (Bessis et al., 2002; Tsuchiya et al., 2002). A fruitful strategy for generating antagonists at both mGlu and GABAB receptors has therefore been to synthesize analogues with side chains, that hinder such closure (Madsen et al., 2005). Seen in this light, it is surprising that none of the tested compounds are antagonists at the human GPRC6A. Furthermore, it is evenly surprising that we identify such a large number of structurally diverse agonists, and it underlines that the ligand-binding pocket of GPRC6A can accommodate much larger structures than the related family C GPCRs in general, and still attain domain closure and receptor activation. Undoubtedly, the design of antagonists for GPRC6A represents a real challenge for medicinal chemists, and obviously calls for identification of residues in the receptor protein interacting with the distal basic side chain of these ligands, hence allowing refinement of the homology model and a more rational ligand design approach. These tasks are currently being addressed in our laboratory.
As discussed above, the compounds tested in this study were chosen for their similarities with basic L-amino acids known to be agonists at GPRC6A, with the intent of advancing pharmacological knowledge of the receptor. The majority of the analogues are, however, primarily known and used for their activity on NOS and arginase enzymes. However, whether the compounds are inhibitors or substrates of these (Table 1) does not seem to influence the generally observed ability to activate the human GPRC6A. From the NOS or arginase investigator's perspective, this report therefore points to potential difficulties when interpreting data using these compounds in vitro or in vivo. The lack of specificity and unexpected activity of some inhibitors of especially NOS has also been reported by others (Buxton et al., 1993; Scheller et al., 1998; Henningsson et al., 2002). Our finding that these compounds also act at GPRC6A has obvious implications for interpretation of experiments involving both the NOS and arginase systems, since GPRC6A is broadly expressed in mammalian tissues (Wellendorph & Bräuner-Osborne, 2004; Kuang et al., 2005). The shared ability of GPRC6A, NOS and arginase enzymes to bind L-arginine, prompted us to compare primary protein sequences and tertiary protein structure of the L-arginine-binding sites. No significant sequence similarity was found, just as the previously generated three-dimensional GPRC6A homology model (Wellendorph et al., 2005) showed no homology with the L-arginine catalytic sites of the murine cytokine-inducible NOS (Crane et al., 1998) and rat arginase I (Christianson, 2005) crystal structures. No information about the ligand specificity of GPRC6A can thus be learned from the NOS and arginase enzyme fields, or vice versa.
Concerning the physiological role of GPRC6A, it has been argued that the receptor could be expected to be permanently activated under physiological conditions (Civelli, 2005) since the L-arginine concentration in plasma is in the 100 μM range and the observed EC50 value of L-arginine is around 20 μM. The reported EC50 values are in fact generated from a non-physiological chimeric receptor of GPRC6A, and should be verified on a native GPRC6A, but in general, one should always take care when extrapolating potencies obtained in recombinant systems to the in vivo situation. Nonetheless, a discrepancy similar to the one discussed here exists between the plasma concentration of L-arginine and the half-saturating concentration (Km) for NOS. Thus, the NOS enzyme should always be saturated, thereby precluding any effect of varying L-arginine concentrations, while in fact in vivo effects on vasodilatation are still observed in response to exogenous L-arginine. This is referred to as the ‘arginine paradox' (Böger & Bode-Böger, 2001). Possible explanations for this phenomenon are that endogenous inhibitors of NOS, such as ADMA or L-NMMA (Vallance & Leiper, 2004), may competitively block the enzyme, thereby increasing the demand for L-arginine substrate, or that endocrine mechanisms such as L-arginine-dependent release of growth hormone, insulin, glucagon, and prolactin may contribute to vasodilatation independently of NO (Böger & Bode-Böger, 2001). Interestingly, these alternative endocrine mechanisms are largely unaccounted for, and allows for speculation as to a role for GPRC6A in the important vascular regulation imposed by L-arginine. Also, as presented herein, GPRC6A is in fact stimulated by the endogenous compounds, ADMA, L-NMMA, and L-NOHA (Campos et al., 1995; Vallance & Leiper, 2004), known to interfere with physiological effects of L-arginine. As mentioned previously, GPRC6A has also been speculated to monitor the urea cycle, extracellular divalent cations, free amino-acid levels in the blood or cell death, to act as a neurotransmitter receptor or take part in a novel cellular communication system (Civelli, 2005; Kuang et al., 2005; Pi et al., 2005; Wellendorph et al., 2005).
Although the physiological importance of GPRC6A remains elusive, its conservation through species argues for relevance, and studies of its structural requirements in the search for potent and selective ligands contribute to establishing the biology of the receptor.
Acknowledgments
We wish to thank Rasmus P. Clausen for help with compound searches, and Birgitte Nielsen for stability studies of L-NAME. J. Ngai (University of Berkeley, CA, U.S.A.) is gratefully acknowledged for providing us with goldfish 5.24 receptor cDNA. This work was supported by the Danish Medical Research Council, Apotekerfonden of 1991, EU Grant HPAW-CT-2002-80057 (PW), and the Novo Scholarship Programme in Biotechnology and Pharmaceutical Sciences (BC).
Abbreviations
- ADMA
asymmetric L-NG,NG-dimethylarginine
- DFMO
DL-α-difluoromethyl-ornithine
- GPCR
G-protein-coupled receptor
- GPRC6A
G-protein-coupled receptor, family C, group 6, subtype A
- mGlu
metabotropic glutamate
- L-NAME
L-NG-nitroarginine methyl ester
- L-NIL
L-N6-(1-iminoethyl)-lysine
- L-NIO
L-N5-(1-iminoethyl)-ornithine
- L-NMMA
L-NG-mono-methylarginine
- L-NMMHA
L-NG-mono-methylhomoarginine
- NO
nitric oxide
- L-NOARG
L-NG-nitroarginine
- L-NOHA
L-NG-hydroxyarginine
- L-nor-NOHA
L-NG-hydroxy-nor-arginine
- NOS
nitric oxide synthase
- vinyl-L-NIO
L-N5-(1-imino-3-butenyl)-ornithine
References
- BERTRAND H.O., BESSIS A.S., PIN J.P., ACHER F.C. Common and selective molecular determinants involved in metabotopic glutamate receptor agonist activity. J. Med. Chem. 2002;45:3171–3183. doi: 10.1021/jm010323l. [DOI] [PubMed] [Google Scholar]
- BESSIS A.S., RONDARD P., GAVEN F., BRABET I., TRIBALLEAU N., PRÉZEAU L., ACHER F., PIN J.P. Closure of the Venus flytrap module of mGlu8 receptor and the activation process: insights from mutations converting antagonists into agonists. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11097–11102. doi: 10.1073/pnas.162138699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN E.M., GAMBA G., RICCARDI D., LOMBARDI M., BUTTERS R., KIFOR O., SUN A., HEDIGER M.A., LYTTON J., HEBERT S.C. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- BRÄUNER-OSBORNE H., EGEBJERG J., NIELSEN E.Ø., MADSEN U., KROGSGAARD-LARSEN P. Ligands for glutamate receptors: design and therapeutic prospects. J. Med. Chem. 2000;43:2609–2645. doi: 10.1021/jm000007r. [DOI] [PubMed] [Google Scholar]
- BUXTON I.L., CHEEK D.J., ECKMAN D., WESTFALL D.P., SANDERS K.M., KEEF K.D. NG-nitro L-arginine methyl ester and other alkyl esters of arginine are muscarinic receptor antagonists. Circ. Res. 1993;72:387–395. doi: 10.1161/01.res.72.2.387. [DOI] [PubMed] [Google Scholar]
- BÖGER R.H., BODE-BÖGER S.M. The clinical pharmacology of L-arginine. Annu. Rev. Pharmacol. Toxicol. 2001;41:79–99. doi: 10.1146/annurev.pharmtox.41.1.79. [DOI] [PubMed] [Google Scholar]
- CAMPOS K.L., GIOVANELLI J., KAUFMAN S. Characteristics of the nitric oxide synthase-catalyzed conversion of arginine to N-hydroxyarginine, the first oxygenation step in the enzymic synthesis of nitric oxide. J. Biol. Chem. 1995;270:1721–1728. doi: 10.1074/jbc.270.4.1721. [DOI] [PubMed] [Google Scholar]
- CHAHINE M., BENNETT P.B., GEORGE A.L., JR, HORN R. Functional expression and properties of the human skeletal muscle sodium channel. Pflugers Arch. 1994;427:136–142. doi: 10.1007/BF00585952. [DOI] [PubMed] [Google Scholar]
- CHRISTIANSON D.W. Arginase: structure, mechanism, and physiological role in male and female sexual arousal. Acc. Chem. Res. 2005;38:191–201. doi: 10.1021/ar040183k. [DOI] [PubMed] [Google Scholar]
- CIVELLI O. An orphan receptor adopted by a family of transmitters. Mol. Pharmacol. 2005;67:583–584. doi: 10.1124/mol.104.010348. [DOI] [PubMed] [Google Scholar]
- CRANE B.R., ARVAI A.S., GHOSH D.K., WU C., GETZOFF E.D., STUEHR D.J., TAINER J.A. Structure of nitric oxide synthase oxygenase dimer with pterin and substrate. Science. 1998;279:2121–2126. doi: 10.1126/science.279.5359.2121. [DOI] [PubMed] [Google Scholar]
- HENNINGSSON R., SALEHI A., LUNDQUIST I. Role of nitric oxide synthase isoforms in glucose-stimulated insulin release. Am. J. Physiol. Cell Physiol. 2002;283:C296–C304. doi: 10.1152/ajpcell.00537.2001. [DOI] [PubMed] [Google Scholar]
- HERMANS E., CHALLISS R.A. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochem. J. 2001;359:465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUANG D., YAO Y., LAM J., TSUSHIMA R.G., HAMPSON D.R. Cloning and characterization of a family C orphan G-protein coupled receptor. J. Neurochem. 2005;93:383–391. doi: 10.1111/j.1471-4159.2005.03025.x. [DOI] [PubMed] [Google Scholar]
- KUANG D., YAO Y., WANG M., PATTABIRAMAN N., KOTRA L.P., HAMPSON D.R. Molecular similarities in the ligand binding pockets of an odorant receptor and the metabotropic glutamate receptors. J. Biol. Chem. 2003;278:42551–42559. doi: 10.1074/jbc.M307120200. [DOI] [PubMed] [Google Scholar]
- KUNISHIMA N., SHIMADA Y., TSUJI Y., SATO T., YAMAMOTO M., KUMASAKA T., NAKANISHI S., JINGAMI H., MORIKAWA K. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature. 2000;407:971–977. doi: 10.1038/35039564. [DOI] [PubMed] [Google Scholar]
- LUU P., ACHER F., BERTRAND H.O., FAN J., NGAI J. Molecular determinants of ligand selectivity in a vertebrate odorant receptor. J. Neurosci. 2004;24:10128–10137. doi: 10.1523/JNEUROSCI.3117-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUZZI S.D., MARLETTA M.A. L-Arginine analogs as alternate substrates for nitric oxide synthase. Bioorg. Med. Chem. Lett. 2005;15:3934–3941. doi: 10.1016/j.bmcl.2005.05.088. [DOI] [PubMed] [Google Scholar]
- MADSEN U., BRÄUNER-OSBORNE H., GREENWOOD J.R., JOHANSEN T.N., KROGSGAARD-LARSEN P., LILJEFORS T., NIELSEN M., FRØLUND B.GABA and glutamate receptor ligands and ther therapeutic potential in CNS disorders Drug Discovery Handbook 2005New York: Wiley; 797–907.ed. Gad, S.C. pp [Google Scholar]
- NELSON G., CHANDRASHEKAR J., HOON M.A., FENG L., ZHAO G., RYBA N.J., ZUKER C.S. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- NELSON G., HOON M.A., CHANDRASHEKAR J., ZHANG Y., RYBA N.J., ZUKER C.S. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- PI M., FABER P., EKEMA G., JACKSON P.D., TING A., WANG N., FONTILLA-POOLE M., MAYS R.W., BRUNDEN K.R., HARRINGTON J.J., QUARLES L.D. Identification of a novel extracellular cation sensing G-protein coupled receptor. J. Biol. Chem. 2005;280:40201–40209. doi: 10.1074/jbc.M505186200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIN J.P., GALVEZ T., PREZEAU L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol. Ther. 2003;98:325–354. doi: 10.1016/s0163-7258(03)00038-x. [DOI] [PubMed] [Google Scholar]
- PIN J.P., KNIAZEFF J., GOUDET C., BESSIS A.S., LIU J., GALVEZ T., ACHER F., RONDARD P., PREZEAU L. The activation mechanism of class-C G-protein coupled receptors. Biol. Cell. 2004;96:335–342. doi: 10.1016/j.biolcel.2004.03.005. [DOI] [PubMed] [Google Scholar]
- PROSKURYAKOV S.Y., KONOPLYANNIKOV A.G., SKVORTSOV V.G., MANDRUGIN A.A., FEDOSEEV V.M. Structure and activity of NO synthase inhibitors specific to the L-arginine binding site. Biochemistry (Mosc.) 2005;70:8–23. [PubMed] [Google Scholar]
- ROBERTSON C.A., GREEN B.G., NIEDZWIECKI L., HARRISON R.K., GRANT S.K. Effect of nitric oxide synthase substrate analog inhibitors on rat liver arginase. Biochem. Biophys. Res. Commun. 1993;197:523–528. doi: 10.1006/bbrc.1993.2510. [DOI] [PubMed] [Google Scholar]
- SCHELLER M., BLOBNER M., VON LOEWENICH C., SCHNECK H., STADLER J., FRANKE C., KOCHS E. The NO synthase inhibitors L-Name and L-NMMA, but not L-arginine, block the mammalian nicotinic acetylcholine receptor channel. Toxicol. Lett. 1998;100–101:109–113. doi: 10.1016/s0378-4274(98)00173-8. [DOI] [PubMed] [Google Scholar]
- SOUTHAN G.J., SZABÓ C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem. Pharmacol. 1996;51:383–394. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]
- SPECA D.J., LIN D.M., SORENSEN P.W., ISACOFF E.Y., NGAI J., DITTMAN A.H. Functional identification of a goldfish odorant receptor. Neuron. 1999;23:487–498. doi: 10.1016/s0896-6273(00)80802-8. [DOI] [PubMed] [Google Scholar]
- TSUCHIYA D., KUNISHIMA N., KAMIYA N., JINGAMI H., MORIKAWA K. Structural views of the ligand-binding cores of a metabotropic glutamate receptor complexed with an antagonist and both glutamate and Gd3+ Proc. Natl. Acad. Sci. U.S.A. 2002;99:2660–2665. doi: 10.1073/pnas.052708599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALLANCE P., LEIPER J. Cardiovascular biology of the asymmetric dimethylarginine:dimethylarginine dimethylaminohydrolase pathway. Arterioscler. Thromb. Vasc. Biol. 2004;24:1023–1030. doi: 10.1161/01.ATV.0000128897.54893.26. [DOI] [PubMed] [Google Scholar]
- WELLENDORPH P., BRÄUNER-OSBORNE H. Molecular cloning, expression, and sequence analysis of GPRC6A, a novel family C G-protein coupled receptor. Gene. 2004;335:37–46. doi: 10.1016/j.gene.2004.03.003. [DOI] [PubMed] [Google Scholar]
- WELLENDORPH P., HANSEN K.B., BALSGAARD A., GREENWOOD J.R., EGEBJERG J., BRÄUNER-OSBORNE H. Deorphanization of GPRC6A: a promiscuous L-α-amino acid receptor with preference for basic amino acids. Mol. Pharmacol. 2005;67:589–597. doi: 10.1124/mol.104.007559. [DOI] [PubMed] [Google Scholar]
- YOKOI I., KABUTO H., HABU H., INADA K., TOMA J., MORI A. Structure–activity relationships of arginine analogues on nitric oxide synthase activity in the rat brain. Neuropharmacology. 1994;33:1261–1265. doi: 10.1016/0028-3908(94)90025-6. [DOI] [PubMed] [Google Scholar]